A hungry nanoparticle that enters your body and eats away at your insides sounds like a nightmare straight out of a Michael Crichton novel. In fact, it could be a future defense against heart attacks, strokes, and potentially other fatal diseases — as strange as that might initially sound.
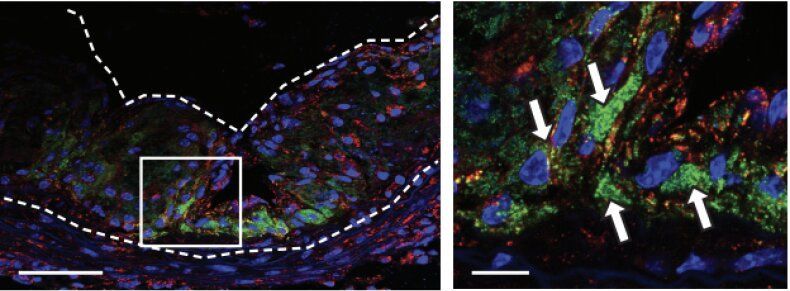
Developed by scientists at Michigan State and Stanford universities, the innovative new “Trojan Horse” nanoparticle works by munching away portions of the plaques responsible for heart attacks. In a proof-of-concept demonstration, the researchers recently showed that their specially developed nanoparticle is able to accurately home in on atherosclerotic plaque, which is responsible for atherosclerosis, one of the leading causes of death in the United States.
“What the nanotherapy does is it enters inflammatory monocytes [a type of white blood cell] in the blood, and carries them into the plaque — hence the ‘Trojan Horse’ label — where they become macrophages, and stimulatesthose and other macrophages in plaque to devour cellular debris,” Bryan Smith, associate professor of biomedical engineering at MSU, told Digital Trends. “This ‘taking out the trash’ attribute stabilizes the plaque with minimal side effects.”