O,.o! Amazing 👏🙀😮
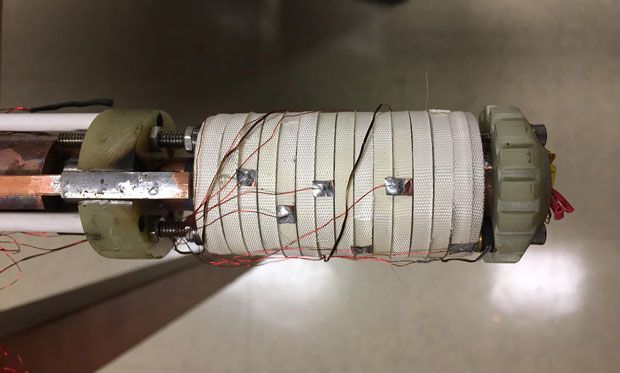
A new multicomponent, partially-superconducting electromagnet—currently the world’s strongest DC magnet of any kind—is poised to reveal a path to substantially stronger magnets still. The new magnet technology could help scientists study many other phenomena including nuclear fusion, exotic states of matter, “shape-shifting” molecules, and interplanetary rockets, to name a few.
The National High Magnetic Field Laboratory in Tallahassee, Florida is home to four types of advanced, ultra-strong magnets. One supports magnetic resonance studies. Another is configured for mass spectrometry. And a different type produces the strongest magnetic fields in the world. (Sister MagLab campuses at the University of Florida and Los Alamos National Laboratory provide three more high-capacity magnets for other fields of study.)
It’s that last category on the Tallahassee campus—world’s strongest magnet—that the latest research is attempting to complement. The so-called MagLab DC Field Facility, in operation since 1999, is nearing a limit in the strength of magnetic fields it can produce with its current materials and technology.