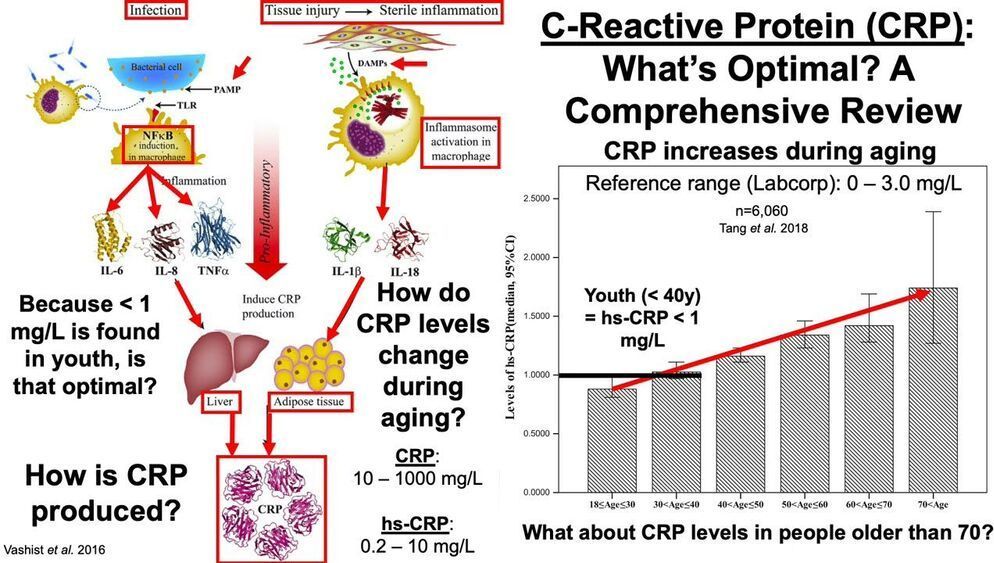
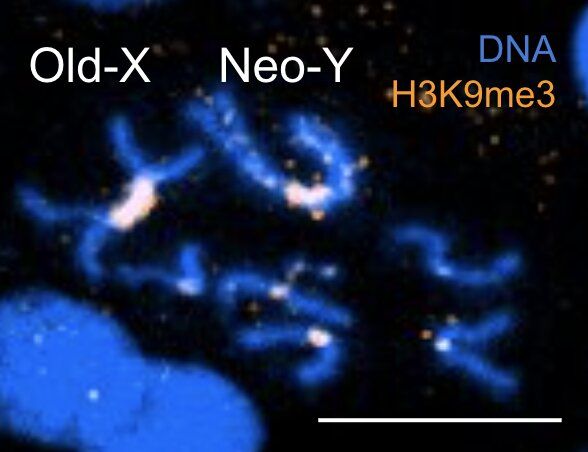
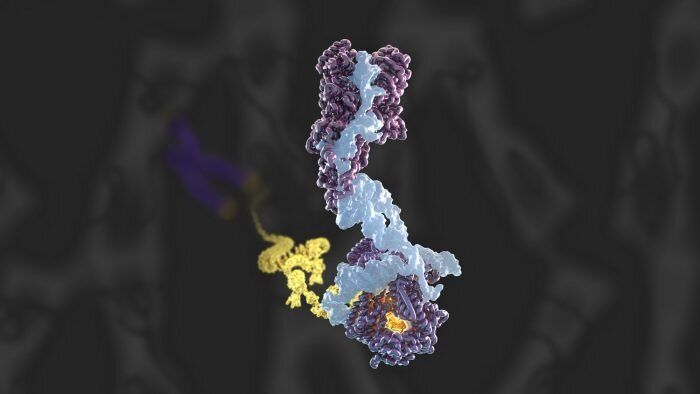
The most interesting part is a longevity escape velocity answer starting at 12:19 and going to 16:30.
Join Aubrey de Grey, Ph.D., Sergey Young, and Sourav Sinha as they talk about how our understanding of aging has developed in the last two decades. They will discuss:
- 7 Mechanisms of Aging.
- Longevity Escape Velocity.
- Human lifespan extension options we have today.
Aubrey de Grey is a biomedical gerontologist and the Chief Science Officer of the SENS Research Foundation. He is editor-in-chief of the academic journal Rejuvenation Research, author of The Mitochondrial Free Radical Theory of Aging (1999), and co-author of Ending Aging (2007)
Sergey Young is the founder of Longevity Vision Fund, XPRIZE Innovation Board member, one of Top-100 Longevity Leaders, and a Forbes Tech Council contributor.
The event is co-hosted by Sourav Sinha. Sourav is a biotech entrepreneur recognized by Forbes 30 Under 30 for disrupting the healthcare space. He is also a World Economic Forum Global Shaper.
___________________________________________
Follow Sergey Young on https://sergeyyoung.com
Purchase “The Science and Technology of Growing Young” at https://amzn.to/3sP8FXA