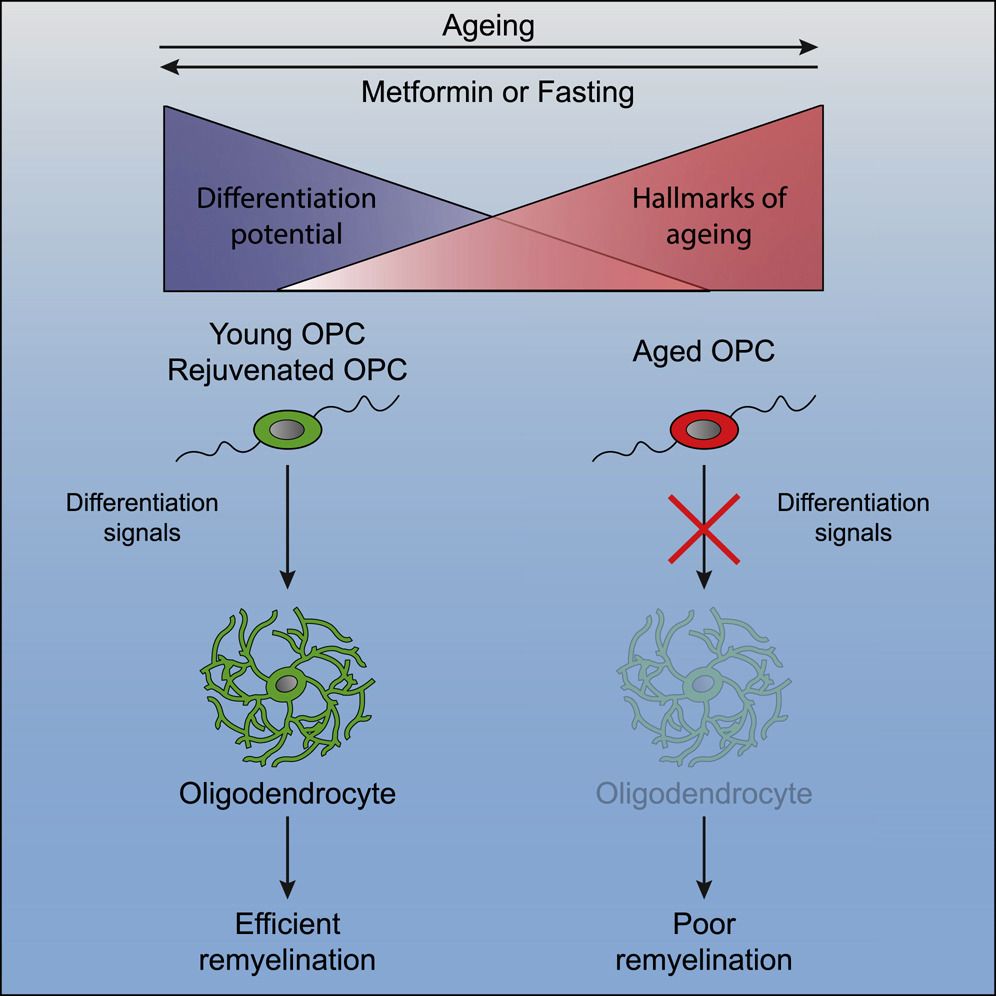
The blind mole rat (Spalax) is a wild, long‐lived rodent that has evolved mechanisms to tolerate hypoxia and resist cancer. Previously, we demonstrated high DNA repair capacity and low DNA damage in Spalax fibroblasts following genotoxic stress compared with rats. Since the acquisition of senescence‐associated secretory phenotype (SASP) is a consequence of persistent DNA damage, we investigated whether cellular senescence in Spalax is accompanied by an inflammatory response. Spalax fibroblasts undergo replicative senescence (RS) and etoposide‐induced senescence (EIS), evidenced by an increased activity of senescence‐associated beta‐galactosidase (SA‐β‐Gal), growth arrest, and overexpression of p21, p16, and p53 mRNAs. Yet, unlike mouse and human fibroblasts, RS and EIS Spalax cells showed undetectable or decreased expression of the well‐known SASP factors: interleukin‐6 (IL6), IL8, IL1α, growth‐related oncogene alpha (GROα), SerpinB2, and intercellular adhesion molecule (ICAM‐1). Apparently, due to the efficient DNA repair in Spalax, senescent cells did not accumulate the DNA damage necessary for SASP activation. Conversely, Spalax can maintain DNA integrity during replicative or moderate genotoxic stress and limit pro‐inflammatory secretion. However, exposure to the conditioned medium of breast cancer cells MDA‐MB‐231 resulted in an increase in DNA damage, activation of the nuclear factor κB (NF‐κB) through nuclear translocation, and expression of inflammatory mediators in RS Spalax cells. Evaluation of SASP in aging Spalax brain and intestine confirmed downregulation of inflammatory‐related genes. These findings suggest a natural mechanism for alleviating the inflammatory response during cellular senescence and aging in Spalax, which can prevent age‐related chronic inflammation supporting healthy aging and longevity.