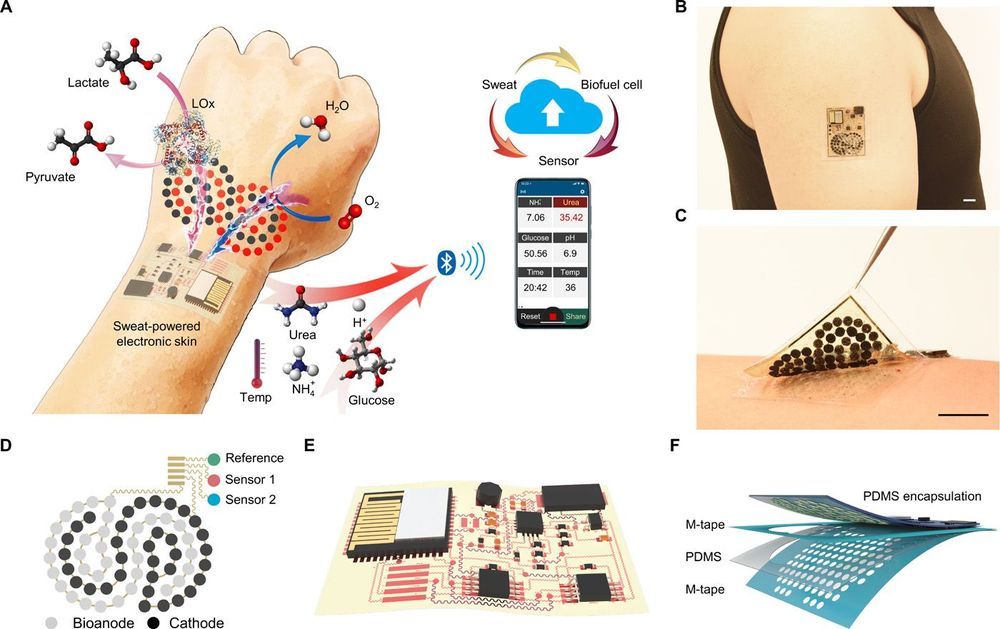
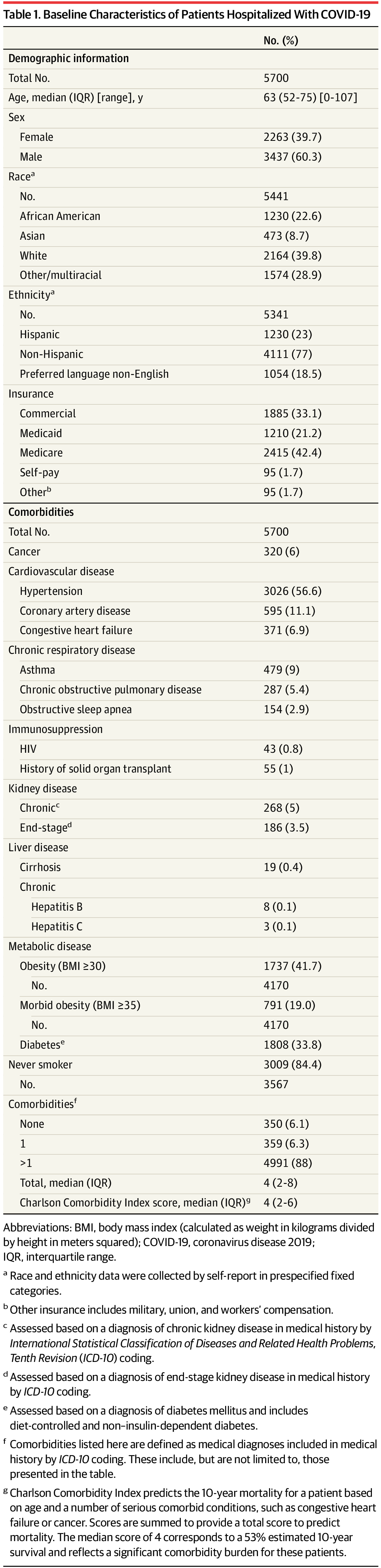
In the constant battle against the spread of infectious diseases, scientists are continually on the hunt for new weapons that specifically target pathogenic microbes. Now, investigators from the Center for Radiological Research at Columbia University Irving Medical Center (CUIMC) believe they may have found a new, low-cost solution to eradicating airborne viruses in indoor public spaces. The research team found that continuous low doses of far ultraviolet C (far-UVC) light can kill airborne flu viruses without harming human tissues. The findings from the new study—published today in Scientific Reports in an article entitled “Far-UVC Light: A New Tool to Control the Spread of Airborne-Mediated Microbial Diseases”—suggests that use of overhead far-UVC light in hospitals, doctors’ offices, schools, airports, airplanes, and other public spaces could provide a powerful check on seasonal influenza epidemics, as well as influenza pandemics.
Scientists have known for decades that broad-spectrum UVC light, which has a wavelength of between 200 to 400 nanometers (nm), is highly effective at killing bacteria and viruses by destroying the molecular bonds that hold their DNA together. This conventional UV light is routinely used to decontaminate surgical equipment.
“Unfortunately, conventional germicidal UV light is also a human health hazard and can lead to skin cancer and cataracts, which prevents its use in public spaces,” explained senior study investigator David Brenner, Ph.D., director of the Center for Radiological Research and professor at CUIMC.