SANTIAGO (Reuters) — The coronavirus has landed in Antarctica, the last continent previously free from COVID-19, Chile’s military said this week, as health and army officials scrambled to clear out and quarantine staff from a remote research station surrounded by ocean and icebergs.
Category: health
Unmanned Aerial Vehicles (UAV), commonly referred to as drones, may prove to be a valuable tool in the battle against pandemics like COVID-19. Researchers at the University of Calgary, the Southern Alberta Institute of Technology (SAIT), Alberta Health Services (AHS) and Alberta Precision Laboratories (APL) are partnering with the Stoney Nakoda Nations (SNN) to deliver medical equipment and test kits for COVID-19 to remote areas, and to connect these communities to laboratories more quickly using these remotely piloted aircraft.
Access for all
“We know that testing for COVID-19 is one of our most effective tools against its spread. Many remote communities in Canada do not have easy access to testing centres and medical supplies to support rapid testing and containment. Drones can help us respond to that need,” says Dr. John Conly, MD, medical director of the W21C Research and Innovation Centre at the Cumming School of Medicine (CSM) and co-principal investigator on the project.
Bats are the second most diverse mammalian group, playing keystone roles in ecosystems but also act as reservoir hosts for numerous pathogens. Due to their colonial habits which implies close contacts between individuals, bats are often parasitized by multiple species of micro-and macroparasites. The particular ecology, behavior, and environment of bat species may shape patterns of intra-and interspecific pathogen transmission, as well as the presence of specific vectorial organisms. This review synthetizes information on a multi-level parasitic system: bats, bat flies and their microparasites. Bat flies (Diptera: Nycteribiidae and Streblidae) are obligate, hematophagous ectoparasites of bats consisting of ~500 described species. Diverse parasitic organisms have been detected in bat flies including bacteria, blood parasites, fungi, and viruses, which suggest their vectorial potential. We discuss the ecological epidemiology of microparasites, their potential physiological effects on both bats and bat flies, and potential research perspectives in the domain of bat pathogens. For simplicity, we use the term microparasite throughout this review, yet it remains unclear whether some bacteria are parasites or symbionts of their bat fly hosts.
Bats are the second most diverse mammalian group after rodents, with ~1390 recognized species across 227 genera (1). Many bat species play keystone roles in ecosystems, where they are essential to pollination, seed dispersal, and pest control (2). Several studies have also highlighted their prominent role as pathogen-reservoirs (3, 4); viruses being the best studied due to their potential as human pathogens (3, 5 – 8). Bats host more viruses per species than rodents, making them an interesting system for both disease ecology and public health research (4, 9).
Bacteria (such as Bartonella spp. and Borrelia spp.) and protozoans (such as Trypanosoma spp. and Plasmodium spp.) have also been detected in bats (8, 10, 11). In recent years, bat-associated Bartonella genotypes have been found in humans, indicating the public health importance of this parasite in bats (12 – 14). Bartonella and other pathogen transmission from bats to humans may occur through religious activities in caves, bat consumption or contact with contaminated products (12, 15). There are documented cases of bat-specific ectoparasites biting humans (16, 17), increasing the potential of bat-born pathogen transmission. Additionally, bat-associated pathogen, such as Trypanosoma cruzi genotype has also been found in humans (18).
MIT anthropologist Amy Moran-Thomas reflects on the deep connection between planetary and human well-being.
When anthropologist Amy Moran-Thomas first went to Belize to begin ethnographic research in 2008, she planned to chronicle human health concerns, focusing on diabetes. Then she learned that local diets contributing to such chronic conditions were changing, in part due to losses in ocean food webs, and kept hearing stories about how local plants were in trouble.
“Listening and trying to learn from what people were saying, over the years I came to see human health and planetary health as deeply interconnected,” says Moran-Thomas, the Morrison Hayes Career Development Associate Professor of Anthropology at MIT. “When I think of health now, I think of disarray in bigger ecosystems and infrastructures that’s also landing in human bodies.”
Each day this week, we will be providing updates on a fictional impact scenario playing out at the International Planetary Science Conference in College Park, Maryland. This scenario is designed to help key decision makers practice for a real asteroid impact. Currently, there is no known asteroid with a significant probability of impacting Earth in the next century. Day 5: What Was This Exercise All About? This week at the 2019 Planetary Defense Conference, conference participants were tasked with responding to a hypothetical asteroid impact scenario in which they have eight years to stop an asteroid on a collision course with Earth. Every day, the audience heard updates — at one point, they weren’t sure whether the 140–260-meter-wide (500−850 feet) asteroid was actually going to hit Earth. Once they found out it was on a certain collision, NASA and space agencies around the world decided to send a fleet of kinetic impactors to deflect the asteroid. The kinetic impactors hit the asteroid…but ended up splitting off a chunk, which, on Day 4 (four years from impact), again was headed towards Earth.
While headlines routinely report on “close shaves” and “near-misses” when near-Earth objects (NEOs) such as asteroids or comets pass relatively close to Earth, the real work of preparing for the possibility of a NEO impact with Earth goes on mostly out of the public eye.
AUSTIN, Texas — Producing clean water at a lower cost could be on the horizon after researchers from The University of Texas at Austin and Penn State solved a complex problem that had baffled scientists for decades, until now.
Desalination membranes remove salt and other chemicals from water, a process critical to the health of society, cleaning billions of gallons of water for agriculture, energy production and drinking. The idea seems simple — push salty water through and clean water comes out the other side — but it contains complex intricacies that scientists are still trying to understand.
The research team, in partnership with DuPont Water Solutions, solved an important aspect of this mystery, opening the door to reduce costs of clean water production. The researchers determined desalination membranes are inconsistent in density and mass distribution, which can hold back their performance. Uniform density at the nanoscale is the key to increasing how much clean water these membranes can create.
Ralph Baric, PhD, is the William R. Kenan, Jr. Distinguished Professor in the Department of Epidemiology and Professor in the Department of Microbiology and Immunology. He is a Harvey Weaver Scholar from the National Multiple Sclerosis Society and an Established Investigator Awardee from the American Heart Association. In addition, he is a World Technology Award Finalist and a fellow of the American Association for Microbiology. He has spent the past three decades as a world leader in the study of coronaviruses and is responsible for UNC-Chapel Hill’s world leadership in coronavirus research. For these past three decades, Dr. Baric has warned that the emerging coronaviruses represent a significant and ongoing global health threat, particularly because they can jump, without warning, from animals into the human population, and they tend to spread rapidly.
The Baric Lab uses coronaviruses as models to study the genetics of RNA virus transcription, replication, persistence, pathogenesis, genetics and cross-species transmission. He has used alphavirus vaccine vectors to develop novel candidate vaccines. Dr. Baric has led the world in recognizing the importance of zoonotic viruses as a potentially rich source of new emerging pathogens in humans, with detailed studies of the molecular, genetic and evolutionary mechanisms that regulate the establishment and dissemination of such a virus within a newly adopted host. Specifically, he works to decipher the complex interactions between the virion and cell surface molecules that function in the entry and cross-species transmission of positive-strand RNA viruses.
In 20172018 and 2019, Dr. Baric was named to Clarivate Analytics’ Highly Cited Researchers list, which recognizes researchers from around the world who published the most widely-cited papers in their field. Also in 2017, he was awarded a grant for more than $6 million from the National Institute of Allergy and Infectious Diseases (NIAID) to accelerate the development of a promising new drug in the fight against deadly coronaviruses, which is currently in clinical trials to reverse COVID-19 disease in humans. In this collaboration, he continued his partnership between the Gillings School and Gilead Sciences Inc. to focus on an experimental antiviral treatment that he had previously shown to prevent the development of severe acute respiratory syndrome coronavirus (SARS-CoV) in mice. The drug also was shown to inhibit MERS-CoV and multiple other coronaviruses (CoV), suggesting that it may actually inhibit all CoV. He continues to work with this drug.
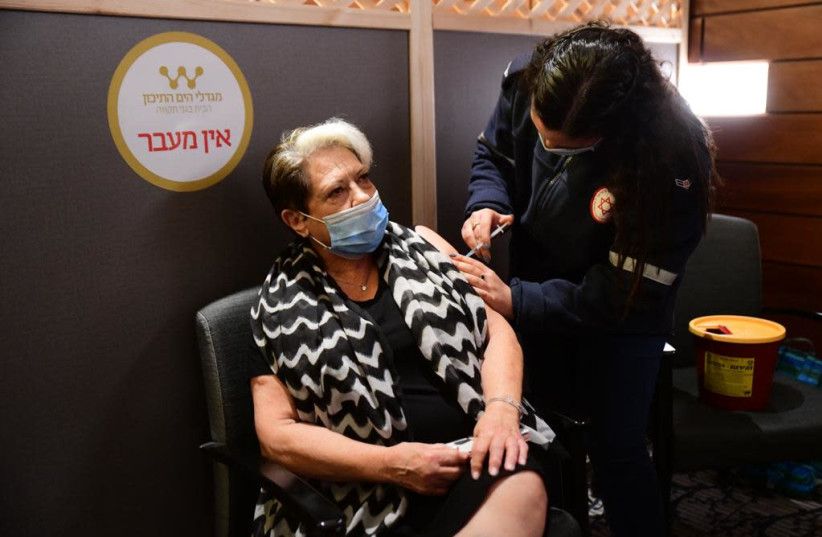
Some 21 residents of a Bat Yam retirement home tested positive for the coronavirus after they were vaccinated but before they had developed antibodies, according to Ynet.
The other 150 residents of the home will be tested for the virus.
Health officials have stressed that the two-dose Pfizer vaccine regimen means that the vaccine is only fully effective about five weeks after the first dose. This means it could take until sometime in February for enough elderly and high-risk people to be vaccinated to help lower the spread of infection and start reopening the economy.
Moreover, the risk of catching coronavirus after the first jab has been confirmed in that some 15000 patients who received the first dose of the vaccine were screened and 428 were confirmed positive for COVID-19 and some 12 people were hospitalized, according to reports. It is possible that some of them were exposed to the virus even before being vaccinated.
On Friday morning, Israel’s one millionth citizen was vaccinated against the novel coronavirus in the presence of Prime Minister Benjamin Netanyahu and Health Minister Yuli Edelstein.
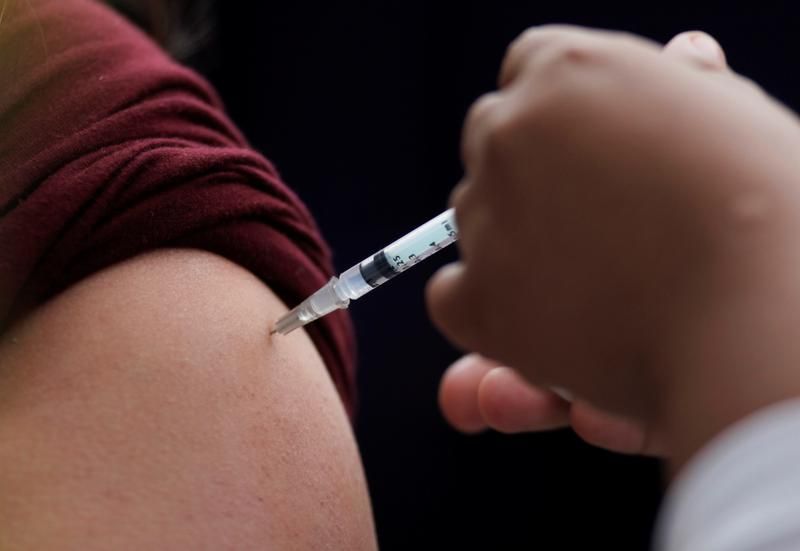
MEXICO CITY (Reuters) — Mexican authorities said they are studying the case of a 32-year-old female doctor who was hospitalized after receiving the Pfizer-BioNTech COVID-19 vaccine.
The doctor, whose name has not been released, was admitted to the intensive care unit of a public hospital in the northern state of Nuevo Leon after she experienced seizures, difficulty breathing and a skin rash.
“The initial diagnosis is encephalomyelitis,” the Health Ministry said in a statement released on Friday night. Encephalomyelitis is an inflammation of the brain and spinal cord.
We strongly believe that only digital health can bring healthcare into the 21st century and make patients the point-of-care.