Using CRISPR, Sandia National Lab researchers are genetically engineering antiviral countermeasures to fight the coronavirus—and potentially future outbreaks.



The results of a clinical trial released today (May 18, 2020) in STEM CELLS Translational Medicine demonstrate how a topical solution made up of stem cells leads to the regrowth of hair for people with a common type of baldness.
Androgenetic alopecia (AGA) — commonly known as male-pattern baldness (female-pattern baldness in women) — is a condition caused by genetic, hormonal and environmental factors. It affects an estimated 50 percent of all men and almost as many women older than 50. While it is not a life-threatening condition, AGA can lower a person’s self-esteem and psychological well-being. There are a few FDA-approved medications to treat hair loss, but the most effective can have side effects such as loss of libido and erectile dysfunction. Therefore, the search continues for a safer, effective treatment.
Adipose tissue-derived stem cells (ADSCs) secrete several growth hormones that help cells develop and proliferate. According to laboratory and experimental studies, growth factors such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF) and platelet-derived growth factor (PDGF) increase the size of the hair follicle during hair development.
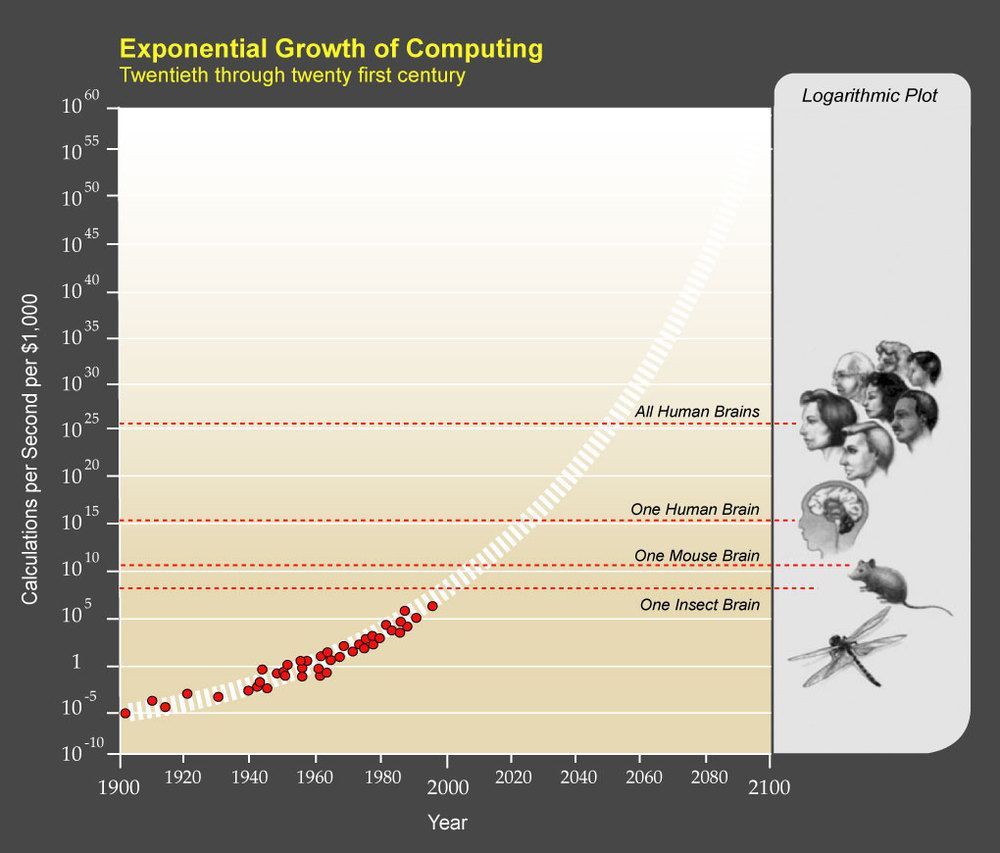
Circa 2010 what someday we could use crispr to develop a biology singularity to find the epigenetics to evolve at lightning speed.
If you’re a sci-fi reader, you are probably familiar with the idea of the “technological singularity”. For the uninitiated, the Singularity is the idea that computational power is increasing so rapidly that soon there will be genuine artificial intelligence that will far surpass humans. Essentially, once you have smarter-than-human computers, they will drive their own advancement and we will no longer be able to comprehend the technology.
We can debate whether the singularity will or will not happen, and what the consequences might be, for a long time, but that’s not the point of this post. This post was inspired by the final chapter in Denialism by Michael Specter. In that chapter, Specter talks about the rapid advancement in biotechnology. Specifically, he points to the rapid increase in computational power and the resulting rapid increase in the speed of genome processing.

Plants can produce energy-rich biomass with the help of light, water and carbon dioxide. This is why they are at the beginning of the food chains. But the carnivorous plants have turned the tables and hunt animals. Insects are their main food source.
A publication in the journal Current Biology now sheds light on the secret life of the green carnivores. The plant scientist Rainer Hedrich and the evolutionary bioinformatician Jörg Schultz, both from Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, and their colleague Mitsujasu Hasebe from the University of Okazaki (Japan) have deciphered and analyzed the genomes of three carnivorous plant species.
They studied the Venus flytrap Dionaea muscipula, which originates from North America, the globally occurring waterwheel plant Aldrovanda vesiculosa and the spoon-leaved sundew Drosera spatulata, which is widely distributed in Asia.
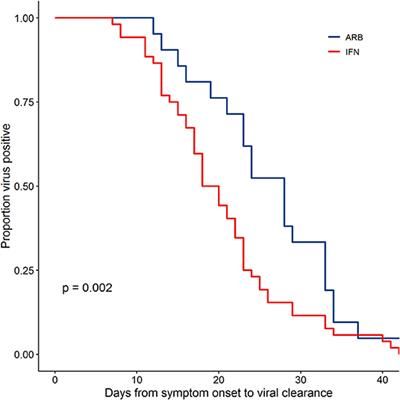
The global pandemic of COVID-19 cases caused by infection with SARS-CoV-2 is ongoing, with no approved antiviral intervention. We describe here the effects of treatment with interferon (IFN)-α2b in a cohort of confirmed COVID-19 cases in Wuhan, China. In this uncontrolled, exploratory study, 77 adults hospitalized with confirmed COVID-19 were treated with either nebulized IFN-α2b (5 mU b.i.d.), arbidol (200 mg t.i.d.) or a combination of IFN-α2b plus arbidol. Serial SARS-CoV-2 testing along with hematological measurements, including cell counts, blood biochemistry and serum cytokine levels, and temperature and blood oxygen saturation levels, were recorded for each patient during their hospital stay. Treatment with IFN-α2b with or without arbidol significantly reduced the duration of detectable virus in the upper respiratory tract and in parallel reduced duration of elevated blood levels for the inflammatory markers IL-6 and CRP. These findings suggest that IFN-α2b should be further investigated as a therapy in COVID-19 cases.
In December 2019, an outbreak of pneumonia was reported in Wuhan, Hubei province, China, resulting from infection with a novel coronavirus (CoV), severe acute respiratory syndrome (SARS)-CoV-2. SARS-CoV-2 is a novel, enveloped betacoronavirus with phylogenetic similarity to SARS-CoV (1). Unlike the coronaviruses HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU, that are pathogenic in humans and are associated with mild clinical symptoms, SARS-CoV-2 resembles both SARS-CoV and Middle East respiratory syndrome (MERS), with the potential to cause more severe disease. A critical distinction is that CoVs that infect the upper respiratory tract tend to cause a mild disease, whereas CoVs that infect both upper and lower respiratory tracts (such as SARS-CoV-2 appears to be) may cause more severe disease. Coronavirus disease (COVID)-19, the disease caused by SARS-CoV-2, has since spread around the globe as a pandemic.
In the absence of a SARS-CoV-2-specific vaccine or an approved antiviral, a number of antivirals are currently being evaluated for their therapeutic effectiveness. Type I IFNs-α/β are broad spectrum antivirals, exhibiting both direct inhibitory effects on viral replication and supporting an immune response to clear virus infection (2). During the 2003 SARS-CoV outbreak in Toronto, Canada, treatment of hospitalized SARS patients with an IFN-α, resulted in accelerated resolution of lung abnormalities (3). Arbidol (ARB) (Umifenovir) (ethyl-6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2 [(phenylthio)methyl]-indole-3-carboxylate hydrochloride monohydrate), a broad spectrum direct-acting antiviral, induces IFN production and phagocyte activation. ARB displays antiviral activity against respiratory viruses, including coronaviruses (4).

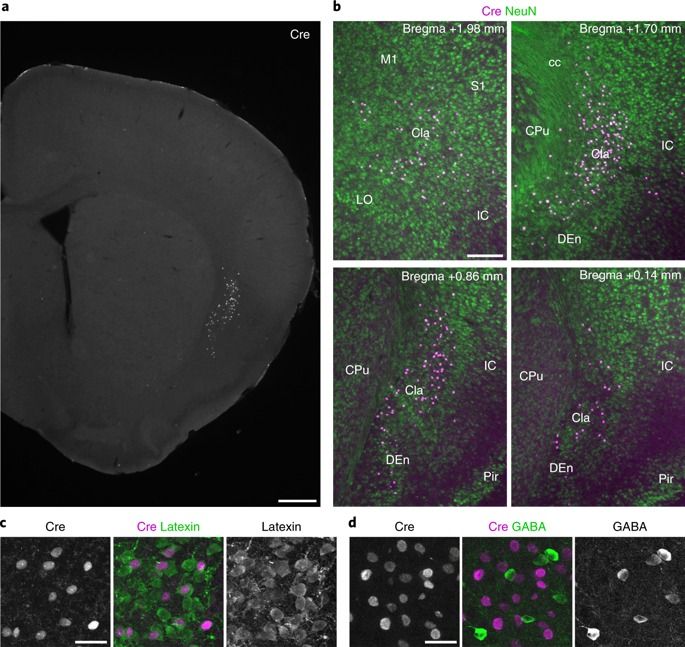
During sleep and awake rest, the neocortex generates large-scale slow-wave (SW) activity. Here, we report that the claustrum coordinates neocortical SW generation. We established a transgenic mouse line that enabled the genetic interrogation of a subpopulation of claustral glutamatergic neurons. These neurons received inputs from and sent outputs to widespread neocortical areas. The claustral neuronal firings mostly correlated with cortical SW activity. In vitro optogenetic stimulation of the claustrum induced excitatory postsynaptic responses in most neocortical neurons, but elicited action potentials primarily in inhibitory interneurons. In vivo optogenetic stimulation induced a synchronized down-state featuring prolonged silencing of neural activity in all layers of many cortical areas, followed by a down-to-up state transition. In contrast, genetic ablation of claustral neurons attenuated SW activity in the frontal cortex. These results demonstrate a crucial role of claustral neurons in synchronizing inhibitory interneurons across wide cortical areas for the spatiotemporal coordination of SW activity.

A group of tiny RNA that should attack the virus causing COVID-19 when it tries to infect the body are diminished with age and chronic health problems, a decrease that likely helps explain why older individuals and those with preexisting medical conditions are vulnerable populations, investigators report.
MicroRNAs play a big role in our body in controlling gene expression, and also are a front line when viruses invade, latching onto and cutting the RNA, the genetic material of the virus, says Dr. Sadanand Fulzele, aging researcher in the Department of Medicine and Center for Healthy Aging at the Medical College of Georgia at Augusta University.
But with age and some chronic medical conditions, the attacking microRNA numbers dwindle, reducing our ability to respond to viruses, says Dr. Carlos M. Isales, co-director of the MCG Center for Healthy Aging and chief of the MCG Division of Endocrinology, Diabetes and Metabolism.
An NC State researcher has developed a new way to get CRISPR/Cas9 into plant cells without inserting foreign DNA. This allows for precise genetic deletions or replacements, without inserting foreign DNA. Therefore, the end product is not a genetically modified organism, or GMO.
CRISPR/Cas9 is a tool that can be used to precisely cut and remove or replace a specific genetic sequence. The Cas9 protein serves as a pair of molecular scissors, guided to the specific genetic target by an easily swapped RNA guide. Basically, it seeks out a specific genetic sequence and, when it finds that sequence, cuts it out. Once the target DNA is snipped, it can be deleted or replaced.
The CRISPR/Cas9 system has tremendous potential for improving crops by changing their genetic code. That does not necessarily mean inserting foreign DNA, but the systems used to deliver CRISPR/Cas9 into a plant’s cells often do, which means the relevant crop is a GMOs undergo through a rigorous evaluation process and many consumers prefer non-GMO products.
If you are interested in age reversal, and you haven’t read Dr David Sinclair (Harvard Medical School) yet, then I’d recommend this research paper.
“Excitingly, new studies show that age-related epigenetic changes can be reversed with interventions such as cyclic expression of the Yamanaka reprogramming factors. This review presents a summary of epigenetic changes that occur in aging, highlights studies indicating that epigenetic changes may contribute to the aging process and outlines the current state of research into interventions to reprogram age-related epigenetic changes.”
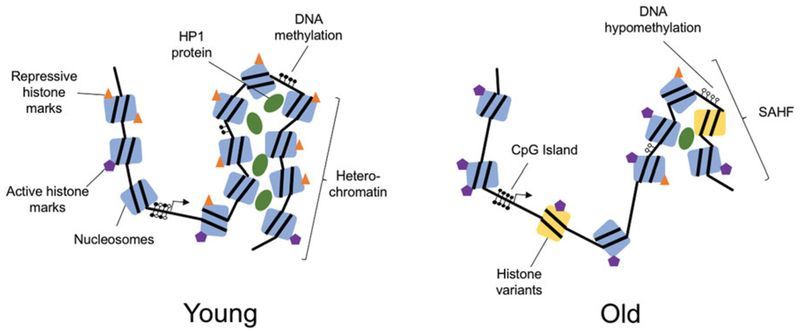
The aging process results in significant epigenetic changes at all levels of chromatin and DNA organization. These include reduced global heterochromatin, nucleosome remodeling and loss, changes in histone marks, global DNA hypomethylation with CpG island hypermethylation, and the relocalization of chromatin modifying factors. Exactly how and why these changes occur is not fully understood, but evidence that these epigenetic changes affect longevity and may cause aging, is growing. Excitingly, new studies show that age-related epigenetic changes can be reversed with interventions such as cyclic expression of the Yamanaka reprogramming factors. This review presents a summary of epigenetic changes that occur in aging, highlights studies indicating that epigenetic changes may contribute to the aging process and outlines the current state of research into interventions to reprogram age-related epigenetic changes.
The term “epigenetics” is thrown around a lot. Originally, it was coined to describe heritable changes that were non-mendelian, but use of the term has evolved. These days, “epigenetics” more generally refers to all non-genomic information storage in cells including gene networks, chromatin structure and post-translational modifications to histones. With aging, there are distinct changes across the epigenome from DNA modifications to alterations in global chromatin organization. But key questions remain unanswered: How and why do these changes occur? Do these changes drive disease and aging? Are they reversible?
Genomic organization is determined by the complex structure of chromatin ( Figure 1 ). The basic unit of chromatin is the nucleosome, which is made up of 147 DNA base pairs wrapped around an octamer of histone proteins. This octamer usually comprises two copies each of H2A, H2B, H3 and H4 (Luger et al. 1997; Hansen 2002). Within nucleosomes, both histones and the DNA itself are subject to a range of chemical modifications that affect the chromatin structure and ultimately the expression of genes. Chromatin falls into one of two major subtypes: euchromatin, in which the chromatin is open and transcriptionally active and heterochromatin, in which the chromatin is tightly closed and transcriptionally silent (Wallrath 1998; Grewal and Moazed 2003). Regulating the epigenetic network are factors that modify chromatin including DNA- and histone-modifying enzymes, transcription factors, and the more recently identified noncoding RNAs (ncRNAs).