Last week, a young woman with sickle cell anemia became the first person in the United States to have her cells altered with CRISPR gene editing technology. Here’s what that means for the future treatment of genetic diseases.



The longest-lived leaves in the plant kingdom can be found only in the harsh, hyperarid desert that crosses the boundary between southern Angola and northern Namibia. A desert is not, of course, the most hospitable place for living things to grow, let alone leafy greens, but the Namib Desert — the world’s oldest, with parts receiving less than 2 inches of precipitation a year — is where Welwitschia calls home. Sign up for The Morning newsletter from the New York Times In Afrikaans, the plant is.

“Balancing that, I clearly state that my goal is not longevity, not even modest longevity. It’s just reversal of diseases of aging, which really is classic medicine. Q: Which takes me to the next question: do we even know how to aim at life extension? I don’t think we do. I think if we get serious aging reversal, it’s something that we can continue to improve on, just like we improved on transportation from the first wheel to rocket ships,” I’ll be honest, I disagree as we have some improvement in humans indicated from TRIM and TAME and plasma filtering. Church’s work is very important though.
Professor of Genetics at Harvard Medical School and one of the most prominent geroscientists, George Church works on gene therapies that can potentially reverse age-related diseases. We had the opportunity to interview this prolific researcher and entrepreneur, who is involved in dozens of startups on topics ranging from the current state of gene therapy to his recent attempt to auction off his genome, one of the first sequenced human genomes in the world, as an NFT.
What have been the successes and the failures of gene therapy in recent years? What do you expect to happen in the next few years?
So, most of the big failures of gene therapy happened at the very beginning, around the year 2000, almost two decades ago, when a couple of people died from an LMO2 oncogene, and one person died from an immune reaction to an adenovirus vector. So, that was 20 years ago. Fast forward to now, and gene therapies are mostly succeeding, hundreds of them are in clinical trials, you have dozens that have been approved by the FDA.
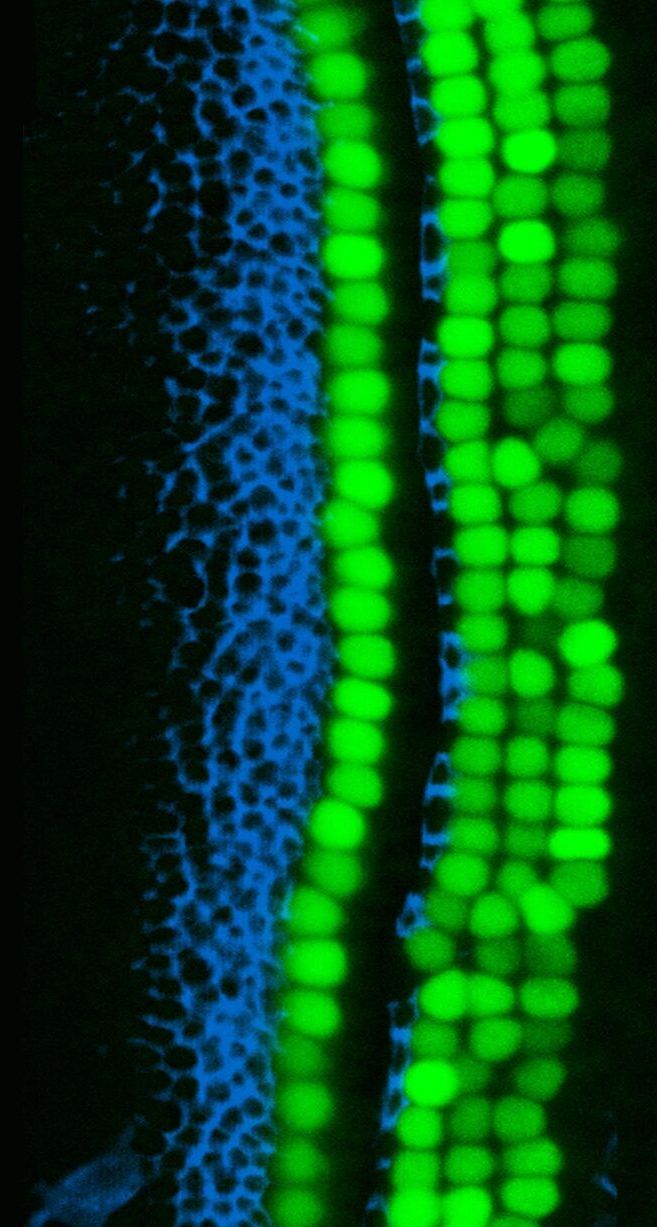
“Our study raises the possibility of using therapeutic drugs, gene editing, or other strategies to make epigenetic modifications that tap into the latent regenerative capacity of inner ear cells as a way to restore hearing,” said Segil. “Similar epigenetic modifications may also prove useful in other non-regenerating tissues, such as the retina, kidney, lung, and heart.”
Scientists from the USC Stem Cell laboratory of Neil Segil have identified a natural barrier to the regeneration of the inner ear’s sensory cells, which are lost in hearing and balance disorders. Overcoming this barrier may be a first step in returning inner ear cells to a newborn-like state that’s primed for regeneration, as described in a new study published in Developmental Cell.
“Permanent hearing loss affects more than 60 percent of the population that reaches retirement age,” said Segil, who is a Professor in the Department of Stem Cell Biology and Regenerative Medicine, and the USC Tina and Rick Caruso Department of Otolaryngology – Head and Neck Surgery. “Our study suggests new gene engineering approaches that could be used to channel some of the same regenerative capability present in embryonic inner ear cells.”
In the inner ear, the hearing organ, which is the cochlea, contains two major types of sensory cells: “hair cells” that have hair-like cellular projections that receive sound vibrations; and so-called “supporting cells” that play important structural and functional roles.

Disrupting a gene responsible for pigments allowed experts in Kobe, Japan to create albino opossum offspring.
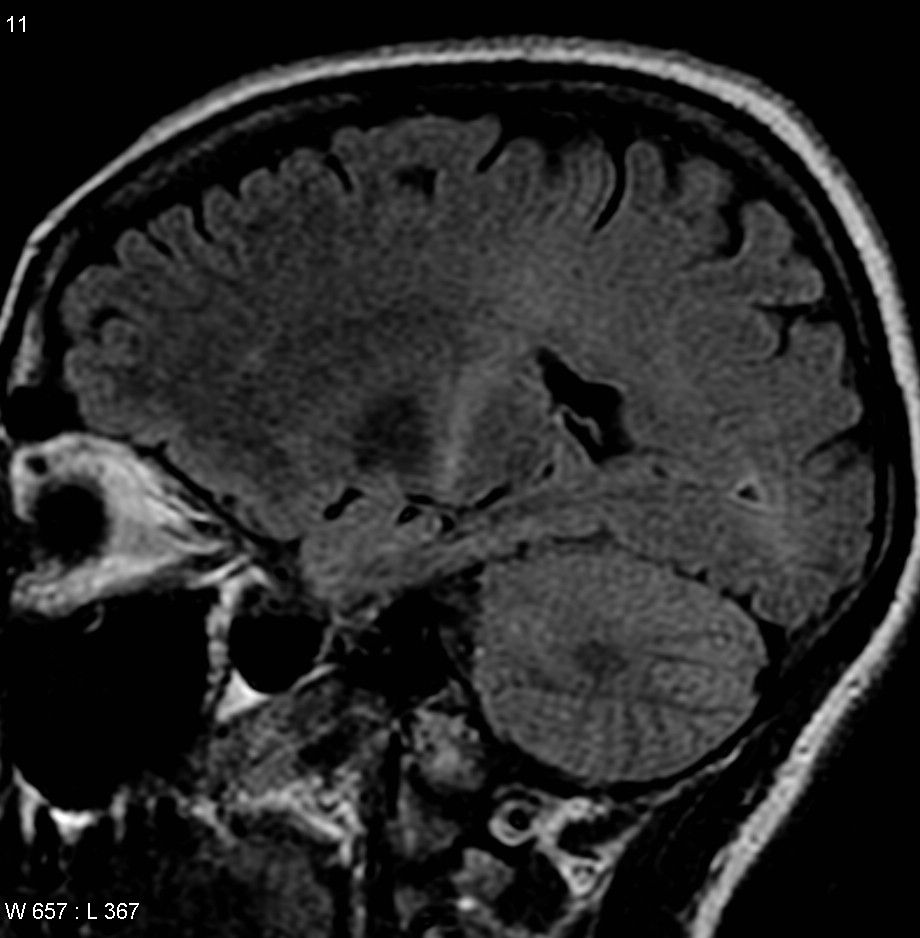
This study builds on an earlier paper by the Rothstein lab that looked at the most common genetic cause of ALS, a mutation in the C9orf72 gene (also referred to as the “C9 mutation”). There, they showed that the C9 mutation produced defects in a structure called the nuclear pore that is responsible for moving proteins and other molecules in and out of the nucleus of cells.
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive and fatal degenerative disease affecting the nerve cells in the brain and spinal cord responsible for controlling voluntary muscle movement. “Sporadic” or non-inherited ALS, accounts for roughly 90% percent of cases, and 10% of cases are due to known genetic mutations. By studying lab-grown neurons derived from skin or blood cells from 10 normal controls, eight with an ALS causing mutation, and 17 with non-inherited ALS, researchers have found a possible starting point for the dysfunction that causes the disease. The study, which was published in Science Translational Medicine, was funded in part by the National Institute for Neurological Disorders and Stroke (NINDS), part of the National Institutes of Health.
Using a library of ALS patient-derived cells, the research team led by Jeffrey Rothstein, M.D., Ph.D., at Johns Hopkins University School of Medicine, Baltimore, developed induced pluripotent stem cell (iPSC)-derived neurons from the patients’ cultured cells to discover a common defect regardless of whether the cell came from persons with inherited or non-inherited ALS. They report that in ALS nerve cells, there is an accumulation of a protein called CHMP7 in the nucleus of cultured nerve cells as well as in ALS samples from the brain region that controls movement. Treatments that decrease the amount of CHMP7 in the cultured cells prevented a series of abnormalities that are characteristic of ALS.
“There is considerable interest in identifying new therapeutic targets for ALS, particularly for the sporadic form of the disorder,” said Amelie Gubitz, Ph.D., program director, NINDS. “Gene-targeting strategies like the one shown here now allow us to move from biological discovery straight to therapy development.”

Despite years of efforts, malaria remains a major health problem. The mosquito-borne parasitic disease sickens more than 200 million people every year and kills more than 400000, many of whom are children.
For the first time, scientists have shown that a new kind of genetic engineering can crash populations of malaria-spreading mosquitoes.
In the landmark study, published Wednesday in the journal Nature Communications, researchers placed the genetically modified mosquitoes in a special laboratory that simulated the conditions in sub-Saharan Africa, where they spread the deadly disease.
The male mosquitoes were engineered with a sequence of DNA known as a “gene drive” that can rapidly transmit a deleterious mutation that essentially wipes out populations of the insects.
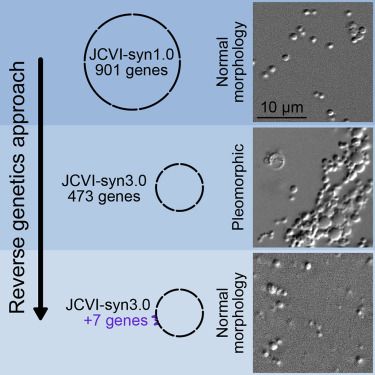
A reverse genetics approach determined that seven genes are required together for normal cell division in a genomically minimal cell; these include two known cell division genes, ftsZ and sepF, a hydrolase of unknown substrate, and four genes that encode membrane-associated proteins of unknown function.
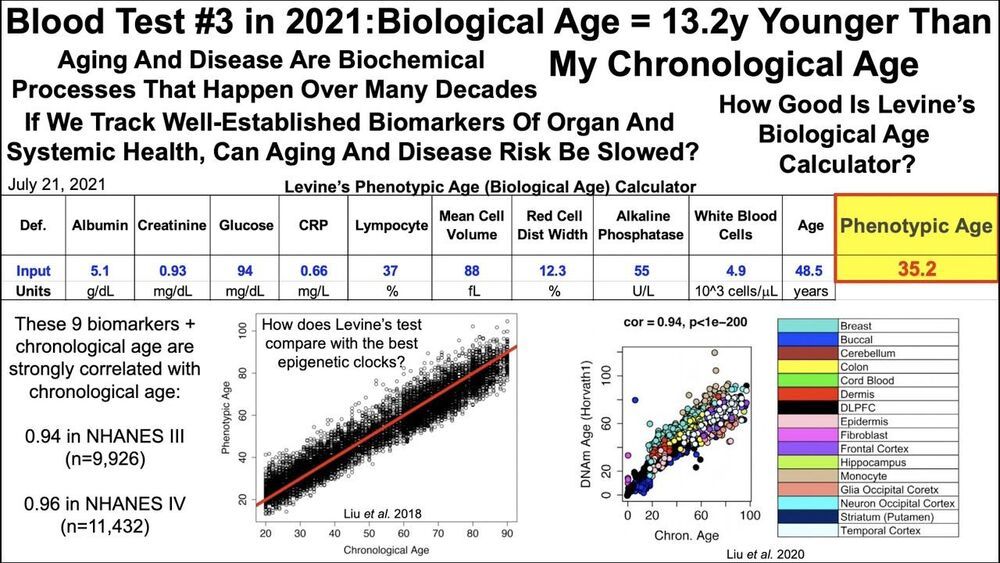
Links to biological age calculators:
Levine’s PhenoAge calculator is embedded as an Excel file:
Aging.ai.
Papers referenced in the video:
A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study.
https://pubmed.ncbi.nlm.nih.gov/30596641/
Underlying features of epigenetic aging clocks in vivo and in vitro.
https://onlinelibrary.wiley.com/doi/full/10.1111/acel.
Population Specific Biomarkers of Human Aging: A Big Data Study Using South Korean, Canadian, and Eastern European Patient Populations.
https://pubmed.ncbi.nlm.nih.gov/29340580/

More TAME! The first part of this has a lot of result data.
Foresight Biotech & Health Extension Meeting sponsored by 100 Plus Capital.
2021 program & apply to join: https://foresight.org/biotech-health-extension-program/
Nir Barzilai, Albert Einstein School of Medicine.
TAME Q&A: Lessons for Progress on Aging.
About Nir Barzilai:
Nir Barzilai, MD, is a Professor in the Department of Endocrinology Medicine and the Department of Genetics at the Albert Einstein College of Medicine. He is also the Ingeborg and Ira Leon Rennert Chair of Aging Research at the Albert Einstein College of Medicine. Dr. Barzilai is the founding director of the Institute for Aging Research at Albert Einstein College of Medicine and the Director of the Nathan Shock Center for Excellence in the Basic Biology of Aging, funded by the National Institutes of Health (NIH); the center is coordinating 80 investigators and six program projects on the biology of aging. He is also the director of the Glenn Center of Excellence in the Biology of Human Aging. He is a chaired professor of medicine and of genetics and a member of the Diabetes Research Center and the divisions of endocrinology and geriatrics. Dr. Barzilai’s interests focus on several basic mechanisms in the biology of aging, including the biological effects of nutrients on extending life and the genetic determinants of life span. His team discovered many longevity gene in humans, and they further characterized the phenotype and genotype of humans with exceptional longevity through NIH awards. He also has an NIH Merit award investigating the metabolic decline that accompanies aging and its impact on longevity. Dr. Barzilai has published more than 270 peer-reviewed papers, reviews and chapters in textbooks. Dr. Barzilai serves on several editorial boards and advisory boards of pharmaceutical and start-up companies, and is a reviewer for numerous journals. A Beeson Fellow for Aging Research, Dr. Barzilai has received many other prestigious awards, including the Senior Ellison Foundation Award, the 2010 Irving S. Wright Award of Distinction in Aging Research, the NIA–Nathan Shock Award and a merit award from the NIA for his contributions in elucidating metabolic and genetic mechanisms of aging and was the 2018 recipient of the IPSEN Longevity award. He is leading the TAME (Targeting/Taming Aging with Metformin) Trial, a multi-center study to prove the concept that multi morbidities of aging can be delayed in humans and change the FDA indications to allow for next generation interventions. He is a founder of CohBar Inc. (now public company) and Medical Advisor for Life Biosciences. He is on the board of AFAR and a founding member of the Academy for Lifespan and Healthspan. He has been featured in major papers, TV programs, and documentaries (TEDx and TEDMED) and has been consulting or presented the promise for targeting aging at The Singapore Prime Minister Office, several International Banks, The Vatican, Pepsico, Milkin Institute, The Economist and Wired Magazine. His book, Age Later: Health Span, Life Span, and the New Science of Longevity, was published by St. Martin’s Press in June of 2020.
Zoom Transcription: https://otter.ai/u/0bz5o2crLQncfxlUkctY6NVzcCg.
Join us:
► Twitter: https://twitter.com/foresightinst.
► Facebook: https://www.facebook.com/foresightinst.
► Instagram: https://www.instagram.com/existentialhope/
► LinkedIn: https://www.linkedin.com/company/foresight-institute.
► Support to join: https://foresight.org/donate/membership/
Foresight Institute advances technologies for the long-term future of life, focusing on molecular machine nanotechnology, biotechnology, and computer science.
Follow us here for videos concerning our programs on Molecular Machines, Biotechnology & Health Extension, Intelligent Cooperation, and Existential Hope.