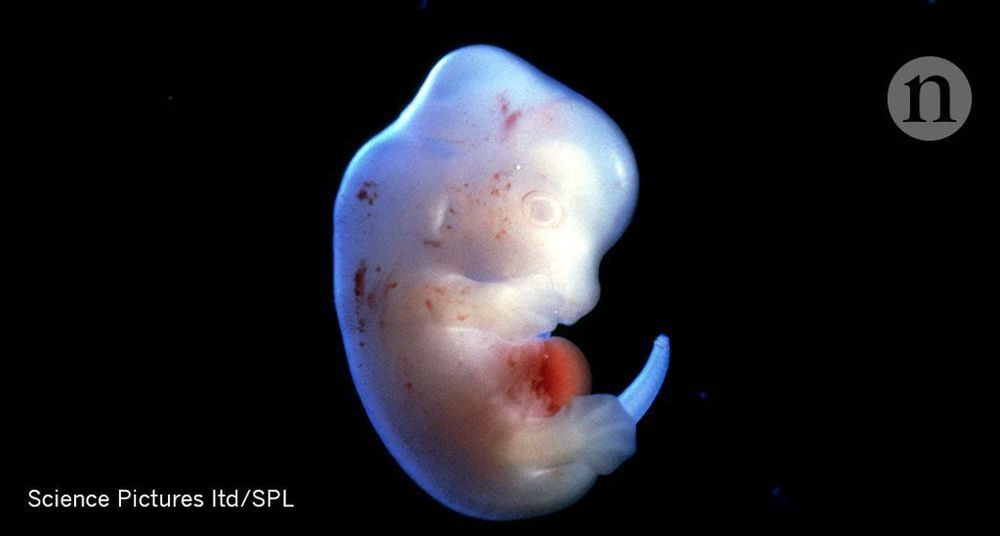
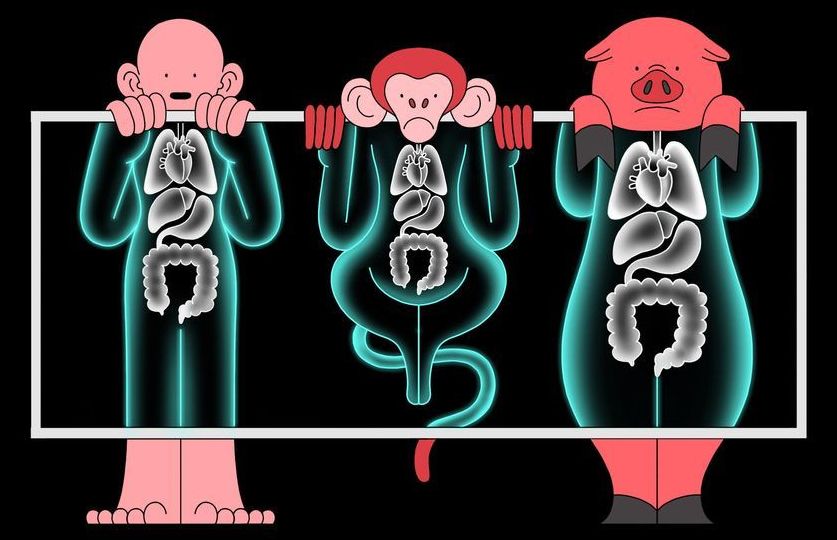
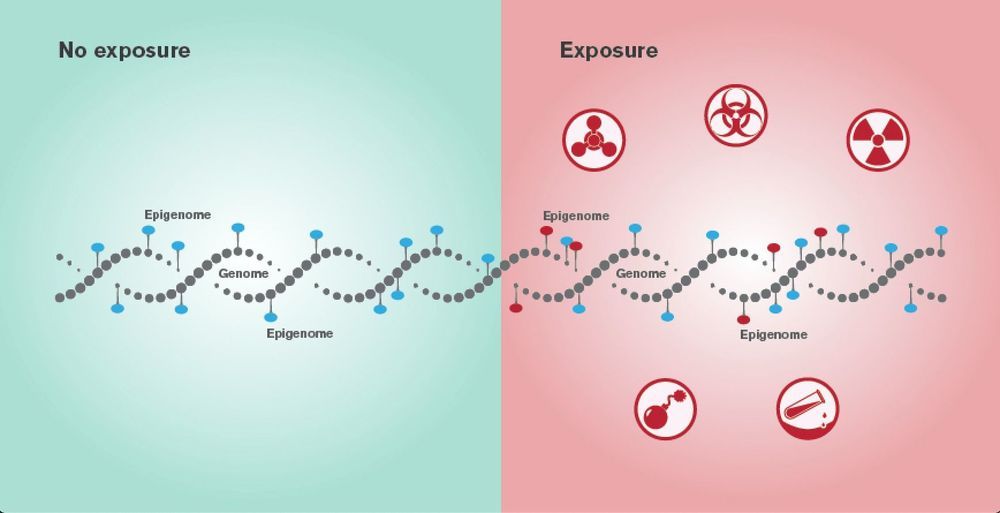
But getting human cells to grow in another species is not easy. Nakauchi and colleagues announced at the 2018 American Association for the Advancement of Science meeting in Austin, Texas that they had put human iPS cells into sheep embryos that had been engineered not to produce a pancreas. But the hybrid embryos, grown for 28 days, contained very few human cells, and nothing resembling organs. This is probably because of the genetic distance between humans and sheep, says Nakauchi.
The research could eventually lead to new sources of organs for transplant, but ethical and technical hurdles need to be overcome.