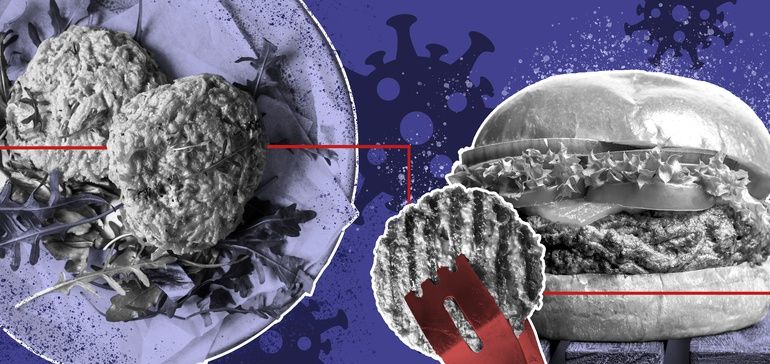
Sales for the category have exploded as the pandemic disrupts business as usual. Is this because people are turning away from animal-based eating, or is it keeping with the segment’s trends?
Category: food
AI, Genetics, and Health-Tech / Wearables — 21st Century Technologies For Healthy Companion Animals.
Ira Pastor ideaXme life sciences ambassador interviews Dr. Angela Hughes, the Global Scientific Advocacy Relations Senior Manager and Veterinary Geneticist at Mars Petcare.
The global petcare industry is significantly expanding, with North America sales alone expected to hit US $300 billion by 2025. And while we may associate the Mars Corporation, the world’s largest candy company, with leading confectionary brands like Milky Way, M&M’s, Skittles, Snickers, Twix, etc. They also happen to be one of the world’s largest companies in pet care as well.
Dr. Angela Hughes, is the Global Scientific Advocacy Relations Senior Manager & Veterinary Geneticist at Mars Petcare. Dr. Hughes is both Doctor of Veterinary Medicine, and a PhD with a focus in Canine Genetics, both from the University of California, Davis. Dr. Hughes also serves as Veterinary Genetics Research Manager of Wisdom Health, a business unit of Mars Petcare, which has developed state-of-the-art genetic tests for companion animals, leading to revolutionary personalized petcare. She also serves as a Veterinary Geneticist of Hughes Veterinary Consulting, focused on small animal and equine genetics and with a special interest in small animal reproduction and pediatrics.
Dr Hughes is published in multiple academic journals, including the Journal of the American Veterinary Medical Association and has contributed chapters for publication in Veterinary Clinics of North America Small Animal Practice: Pediatrics and Large Animal Internal Medicine.
On this ideaXme episode we will hear from Dr. Hughes about:
-Her background — how she developed an interest in veterinary medicine and animal genetics, and how she arrived at Mars Petcare.
-Her role as the senior manager of Global Scientific Advocacy Relations at Mars Petcare.
Scientists find that A. echinatior ants have biomineral armour to help them in battle with other ants and protect them from pathogens. 😃
Ants are pretty organised little creatures. Highly social insects, they know how to forage, build complicated nests, steal your pantry snacks, and generally look after the queens and the colony, all by working together.
Leaf-cutter ants turn that cooperation up several notches. Leaf-cutter ant colonies like Acromyrmex echinatior can contain millions of ants, split into four castes that all have different roles to maintain a garden of fungus that the ants eat.
These farming ants might make a top-tier team of gardeners, but that doesn’t mean they don’t get into the occasional scrap, and living in such large groups usually also means facing an increased risk of pathogens.
10% longer.
Reduced food intake, known as dietary restriction, leads to a longer lifespan in many animals and can improve health in humans. However, the molecular mechanisms underlying the positive effects of dietary restriction are still unclear. Researchers from the Max Planck Institute for Biology of Aging have now found one possible explanation in fruit flies: they identified a protein named Sestrin that mediates the beneficial effects of dietary restriction. By increasing the amount of Sestrin in flies, researchers were able to extend their lifespan and at the same time these flies were protected against the lifespan-shortening effects of a protein-rich diet. The researchers could further show that Sestrin plays a key role in stem cells in the fly gut thereby improving the health of the fly.
The health benefits of dietary restriction have long been known. Recently, it has become clear that restriction of certain food components, especially proteins and their individual building blocks, the amino acids, is more important for the organism’s response to dietary restriction than general calorie reduction. On the molecular level, one particular well-known signaling pathway, named TOR pathway, is important for longevity.
“We wanted to know which factor is responsible for measuring nutrients in the cell, especially amino acids, and how this factor affects the TOR pathway,” explains Jiongming Lu, researcher in the department of Linda Partridge at the Max Planck Institute for Biology of Aging. “We focused on a protein called Sestrin, which was suggested to sense amino acids. However, no one has ever demonstrated amino acid sensing function of Sestrin in a living being.” Therefore, Lu and his colleagues focused on the role of Sestrin in the model organism Drosophila melanogaster, commonly known as fruit fly.
Using drones for agriculture! Technology used to benefit one of the oldest industries. 😃
Designed for use in agriculture, the new DJI T20 is bringing the latest tech to one of the world’s oldest industries 👏 😎.
O,.o Circa 2019
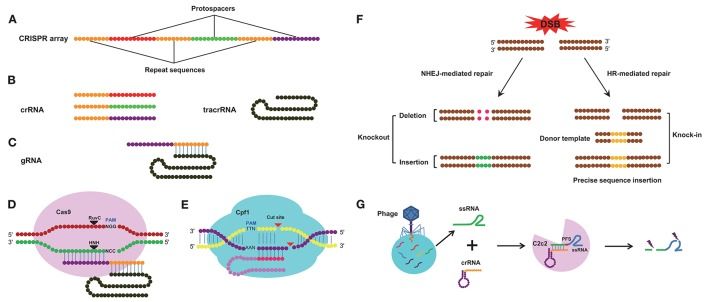
CRISPR/Cas9 is now a household name associated with genetic engineering studies. Through cutting-edge research described in their paper published in Scientific Reports, a team of researchers from Tokyo University of Science, Meiji University, and Tokyo University of Agriculture and Technology, led by Dr Takayuki Arazoe and Prof Shigeru Kuwata, has recently established a series of novel strategies to increase the efficiency of targeted gene disruption and new gene “introduction” using the CRISPR/Cas9 system in the rice blast fungus Pyricularia (Magnaporthe) oryzae. These strategies include quicker (single-step) gene introduction, use of small homologous sequences, and bypassing of certain prerequisite host DNA “patterns” and host component modification.
The team led by Dr Arazoe and Prof Kuwata has devised simple and quick techniques for gene editing (target gene disruption, sequence substitution, and re-introduction of desired genes) using CRISPR/Cas9 in the rice blast fungus Pyricularia (Magnaporthe) oryzae, a type of filamentous fungus. Spurred on by encouraging results, the researchers surmise, “Plants and their pathogens are still coevolving in nature. Exploiting the mutation mechanisms of model pathogenic fungi as a genome editing technique might lead to the development of further novel techniques in genetic engineering.”
The working component of the CRISPR/Cas9 system binds to the target gene region (DNA) and causes a site-specific double-stranded break (DSB) in the DNA. Effective binding of this component requires a certain “motif” or “pattern” called the protospacer-adjacent motif (PAM), which follows downstream of the target gene region.
For more than two decades, I have been working to improve several staple food crops in Africa, including bananas, plantains, cassavas and yams. As principal scientist and a plant biotechnologist at the International Institute for Tropical Agriculture in Nairobi, I aim to develop varieties that are resistant to pests and diseases such as bacterial wilt, Fusarium wilt (caused by the fungus F. oxysporum) and banana streak virus.
[Editor’s note: Abdullahi Tsanni is a freelance science journalist based in Abuja, Nigeria.]
In 2011, my team and I created a set of tools, the only one of its kind in Africa, for changing DNA sequences so that we could develop genetically modified and genome-edited products in sub-Saharan Africa. In 2018, we pioneered the first application of CRISPR gene-editing technology to deactivate banana streak virus in plantains. This technology overcame a major hurdle in banana breeding on the continent, and is the first reported successful use of genome editing to improve bananas.
The honeybee (Apis mellifera) is an important insect pollinator of wild flowers and crops, playing critical roles in the global ecosystem. Additionally, the honeybee serves as an ideal social insect model. Therefore, functional studies on honeybee genes are of great interest. However, until now, effective gene manipulation methods have not been available in honeybees. Here, we reported an improved CRISPR/Cas9 gene-editing method by microinjecting sgRNA and Cas9 protein into the region of zygote formation within 2 hr after queen oviposition, which allows one-step generation of biallelic knockout mutants in honeybee with high efficiency. We first targeted the Mrjp1 gene. Two batches of honeybee embryos were collected and injected with Mrjp1 sgRNA and Cas9 protein at the ventral cephalic side and the dorsal posterior side of the embryos, respectively. The gene-editing rate at the ventral cephalic side was 93.3%, which was much higher than that (11.8%) of the dorsal-posterior-side injection. To validate the high efficiency of our honeybee gene-editing system, we targeted another gene, Pax6, and injected Pax6 sgRNA and Cas9 protein at the ventral cephalic side in the third batch. A 100% editing rate was obtained. Sanger sequencing of the TA clones showed that 73.3% (for Mrjp1) and 76.9% (for Pax6) of the edited current-generation embryos were biallelic knockout mutants. These results suggest that the CRISPR/Cas9 method we established permits one-step biallelic knockout of target genes in honeybee embryos, thereby demonstrating an efficient application to functional studies of honeybee genes. It also provides a useful reference to gene editing in other insects with elongated eggs.
Clustered regularly interspaced short palindromic repeats (CRISPR) and the CRISPR-associated gene Cas9 represent an invaluable system for the precise editing of genes in diverse species. The CRISPR/Cas9 system is an adaptive mechanism that enables bacteria and archaeal species to resist invading viruses and phages or plasmids. Compared with zinc finger nucleases and transcription activator-like effector nucleases, the CRISPR/Cas9 system has the advantage of requiring less time and effort. This efficient technology has been used in many species, including diverse arthropods that are relevant to agriculture, forestry, fisheries, and public health; however, there is no review that systematically summarizes its successful application in the editing of both insect and non-insect arthropod genomes. Thus, this paper seeks to provide a comprehensive and impartial overview of the progress of the CRISPR/Cas9 system in different arthropods, reviewing not only fundamental studies related to gene function exploration and experimental optimization but also applied studies in areas such as insect modification and pest control. In addition, we also describe the latest research advances regarding two novel CRISPR/Cas systems (CRISPR/Cpf1 and CRISPR/C2c2) and discuss their future prospects for becoming crucial technologies in arthropods.
Keywords: CRISPR/Cas9, insects, non-insect arthropods, research progress, prospects.
Genome editing technologies are useful for understanding the functions of target genes in diverse organisms (Segal and Meckler, 2013). Before the CRISPR/Cas9 system was discovered, zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) technologies were used for genome modification; both technologies can be used to design a DNA-binding domain that can effectively recognize and modify virtually any sequence, and both technologies have been widely applied in various fields (Gaj et al., 2013). ZFNs and TALENs, however, require the use of a variety of nucleases, and the off-target effects of nucleases can lead to cellular toxicity. In addition, methods using ZFNs and TALENs are complex and labor-intensive (Kanchiswamy et al., 2016). These two genome-editing systems have been recently replaced by the CRISPR/Cas9 system, which is far more convenient and effective than ZFNs and TALENs (Lander, 2016; Mohanraju et al., 2016; Wang H. et al., 2016; Westra et al.