People who understand evolution are more likely to accept it, study shows. #DarwinDay


People who understand evolution are more likely to accept it, study shows. #DarwinDay
O.o!
The first sequenced genome was that of the 3569-nucleotide single-stranded RNA (ssRNA) bacteriophage MS2. Despite the recent accumulation of vast amounts of DNA and RNA sequence data, only 12 representative ssRNA phage genome sequences are available from the NCBI Genome database (June 2019). The difficulty in detecting RNA phages in metagenomic datasets raises questions as to their abundance, taxonomic structure, and ecological importance. In this study, we iteratively applied profile hidden Markov models to detect conserved ssRNA phage proteins in 82 publicly available metatranscriptomic datasets generated from activated sludge and aquatic environments. We identified 15,611 nonredundant ssRNA phage sequences, including 1015 near-complete genomes. This expansion in the number of known sequences enabled us to complete a phylogenetic assessment of both sequences identified in this study and known ssRNA phage genomes. Our expansion of these viruses from two environments suggests that they have been overlooked within microbiome studies.
Viruses, particularly bacteriophages targeting prokaryotes, are the most diverse biological entities in the biosphere (1, 2). Currently, there are 11,489 genome sequences available in the NCBI (National Center for Biotechnology Information) Viral RefSeq database (version 94). The vast majority of known phage have a double-stranded DNA (dsDNA) genome (3, 4). Recent metagenomic analysis of 145 marine virome sampling sites identified 195,728 DNA viral populations, highlighting that only a fraction of Earth’s viral diversity has been characterized (5). An additional expansion of known phage populations by Roux et al. (6) revealed that not only dsDNA phages but also single-stranded DNA Inoviridae are far more diverse than previously considered. The rapid expansion in viral discovery through metagenomics is enabling a greater understanding of their roles within environments and their evolutionary relationships, which is subsequently causing a revolution in phage taxonomy (7).
Despite the identification of single-stranded RNA (ssRNA) phages over 50 years ago (8), there are few representative sequences available. The International Committee on Taxonomy of Viruses (ICTV) has currently categorized approximately 5500 viruses (9). Yet, their classification only applies to 25 ssRNA phage sequences (complete or partial) across two genera, Levivirus and Allolevivirus, and an additional 32 sequences unclassified below a family taxonomic rank (10). Historically, methods for classifying Leviviridae depended on molecular weight, density, sedimentation, and serological cross-reactivity (11). A subsequent classification method separated the two genera, with the Alloleviviruses containing a fourth unique gene predicted to encode a lysin (12). Recently, an analysis of the evolution origin of all currently known RNA viruses by Wolf et al. (13) suggested that ssRNA phages may actually be two distinct lineages, which they termed Leviviridae and “Levi-like” viruses.

Evolutionary Medicine — Linking human pathology with our past, present, and future evolutionary trajectories — ideaXme (http://radioideaxme.com/) welcomes Prof. Dr. Frank Rühli, Director of the Institute of Evolutionary Medicine, University of Zurich — #Ideaxme #EvolutionaryMedicine #Evolution #Microevolution #Paleopathology #BiologicalAnthropology #ComparativeAnatomy #Mummies #Mummy #Hypercholesterinemia #Diabetes #DrugAddiction #Health #Wellness #Regeneration #Longevity #Aging #IraPastor #Bioquark #Regenerage
Ira Pastor, ideaXme exponential health ambassador, interviews Professor Dr. Frank Rühli, Director of the Institute of Evolutionary Medicine and on the Medical Faculty of University of Zurich, and Founding Director, Chair, Full Professor of Evolutionary Medicine.
Ira Pastor Comments
Famous prominent Russian-American geneticist and evolutionary biologist, Theodosius Dobzhansky, stated in a 1973 essay that Nothing in biology makes sense except in the light of evolution.”
When one applies this principle to medical research, it suggests that if you study only the proximal causes of health and disease (pathophysiology), you get a limited picture, and such dynamics could be better understood within an evolutionary framework.
While traditional biomedical research is often concerned with pathophysiology, it is the relatively new science of evolutionary medicine that seeks to link human pathology with our past, present, and future evolutionary trajectories.
Combining the study of proximal and distal reasons underpinning medical disorders yields a deeper understanding that may help to improve the ways diseases are screened for, treated, or prevented.
Professor Dr. Frank Rühli

The Tasmanian devil (Sarcophilus harrisii), the largest marsupial carnivore, is endangered due to a transmissible facial cancer spread by direct transfer of living cancer cells through biting. Here we describe the sequencing, assembly, and annotation of the Tasmanian devil genome and whole-genome sequences for two geographically distant subclones of the cancer. Genomic analysis suggests that the cancer first arose from a female Tasmanian devil and that the clone has subsequently genetically diverged during its spread across Tasmania. The devil cancer genome contains more than 17,000 somatic base substitution mutations and bears the imprint of a distinct mutational process. Genotyping of somatic mutations in 104 geographically and temporally distributed Tasmanian devil tumors reveals the pattern of evolution and spread of this parasitic clonal lineage, with evidence of a selective sweep in one geographical area and persistence of parallel lineages in other populations.
Circa 2013
In a feat of “molecular time travel,” the researchers resurrected and analyzed the functions of the ancestors of genes that play key roles in modern human reproduction, development, immunity and cancer. By re-creating the same DNA changes that occurred during those genes’ ancient history, the team showed that two mutations set the stage for hormones like estrogen, testosterone and cortisol to take on their crucial present-day roles.
“Changes in just two letters of the genetic code in our deep evolutionary past caused a massive shift in the function of one protein and set in motion the evolution of our present-day hormonal and reproductive systems,” said Joe Thornton, PhD, professor of human genetics and ecology & evolution at the University of Chicago, who led the study.
“If those two mutations had not happened, our bodies today would have to use different mechanisms to regulate pregnancy, libido, the response to stress, kidney function, inflammation, and the development of male and female characteristics at puberty,” Thornton said.

Circa 2011 essentially cancer could help with evolution as it can challenge the immune system to be more strong. Essentially a symbiotic relationship to evolve with it and grow stronger with it then like it can be used as a good thing to make sure that evolution has stronger genetic code.
Evolutionary theories are critical for understanding cancer development at the level of species as well as at the level of cells and tissues, and for developing effective therapies. Animals have evolved potent tumor suppressive mechanisms to prevent cancer development. These mechanisms were initially necessary for the evolution of multi-cellular organisms, and became even more important as animals evolved large bodies and long lives. Indeed, the development and architecture of our tissues were evolutionarily constrained by the need to limit cancer. Cancer development within an individual is also an evolutionary process, which in many respects mirrors species evolution. Species evolve by mutation and selection acting on individuals in a population; tumors evolve by mutation and selection acting on cells in a tissue. The processes of mutation and selection are integral to the evolution of cancer at every step of multistage carcinogenesis, from tumor genesis to metastasis. Factors associated with cancer development, such as aging and carcinogens, have been shown to promote cancer evolution by impacting both mutation and selection processes. While there are therapies that can decimate a cancer cell population, unfortunately, cancers can also evolve resistance to these therapies, leading to the resurgence of treatment-refractory disease. Understanding cancer from an evolutionary perspective can allow us to appreciate better why cancers predominantly occur in the elderly, and why other conditions, from radiation exposure to smoking, are associated with increased cancers. Importantly, the application of evolutionary theory to cancer should engender new treatment strategies that could better control this dreaded disease.
We expect that the public generally views evolutionary biology as a science about the past, with stodgy old professors examining dusty fossils in poorly lit museum basements. Evolution must certainly be a field well-separated from modern medicine and biomedical research, right? If the public makes a connection between evolution and medicine, it is typically in the example of bacteria acquiring antibiotic resistance. But what does evolution have to do with afflictions like heart disease, obesity, and cancer? As it turns out, these diseases are intricately tied to our evolutionary histories, and understanding evolution is essential for preventing, managing and treating these diseases (1, 2). This review will focus on cancer: how evolutionary theories can be used to understand cancer development at the level of species as well as at the level of cells and tissues. We will also discuss the implications and benefits of an evolutionary perspective towards cancer prevention and therapies.
For almost all animals, old age is associated with a general decline in tissue structure and function. This decline is thought to reflect the lack of selective pressure to maintain tissues beyond an age when the animal would be likely to contribute genetically to future generations (3−5). Similarly, there is little selective pressure to limit cancer in old animals who are substantially beyond their reproductive years. For example, while mice can live 2–4 years in the lab, and tend to develop cancer in their second and third years, it is rare to find a mouse greater than 1 year old in the wild. Most wild mice will be dead from other causes, such as cold, hunger, disease or predators, well before the age when cancer would be a likely cause of their demise. Thus, evolution has favored a “breed early, breed often” strategy for mice.

Study reveals interplay of an African bat, a parasite and a virus
Since the emergence of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrom Coronavirus (MERS-CoV) it has become increasingly clear that bats are important reservoirs of CoVs. Despite this, only 6% of all CoV sequences in GenBank are from bats. The remaining 94% largely consist of known pathogens of public health or agricultural significance, indicating that current research effort is heavily biased towards describing known diseases rather than the ‘pre-emergent’ diversity in bats. Our study addresses this critical gap, and focuses on resource poor countries where the risk of zoonotic emergence is believed to be highest. We surveyed the diversity of CoVs in multiple host taxa from twenty countries to explore the factors driving viral diversity at a global scale. We identified sequences representing 100 discrete phylogenetic clusters, ninety-one of which were found in bats, and used ecological and epidemiologic analyses to show that patterns of CoV diversity correlate with those of bat diversity. This cements bats as the major evolutionary reservoirs and ecological drivers of CoV diversity. Co-phylogenetic reconciliation analysis was also used to show that host switching has contributed to CoV evolution, and a preliminary analysis suggests that regional variation exists in the dynamics of this process. Overall our study represents a model for exploring global viral diversity and advances our fundamental understanding of CoV biodiversity and the potential risk factors associated with zoonotic emergence.

Am Nat. 2017 Nov;190:694–706. doi: 10.1086÷693854. Epub 2017 Sep 5.
Biological invasions offer interesting situations for observing how novel interactions between closely related, formerly allopatric species may trigger phenotypic evolution in situ. Assuming that successful invaders are usually filtered to be competitively dominant, invasive and native species may follow different trajectories. Natives may evolve traits that minimize the negative impact of competition, while trait shifts in invasives should mostly reflect expansion dynamics, through selection for colonization ability and transiently enhanced mutation load at the colonization front. These ideas were tested through a large-scale common-garden experiment measuring life-history traits in two closely related snail species, one invasive and one native, co-occurring in a network of freshwater ponds in Guadeloupe. We looked for evidence of recent evolution by comparing uninvaded or recently invaded sites with long-invaded ones.
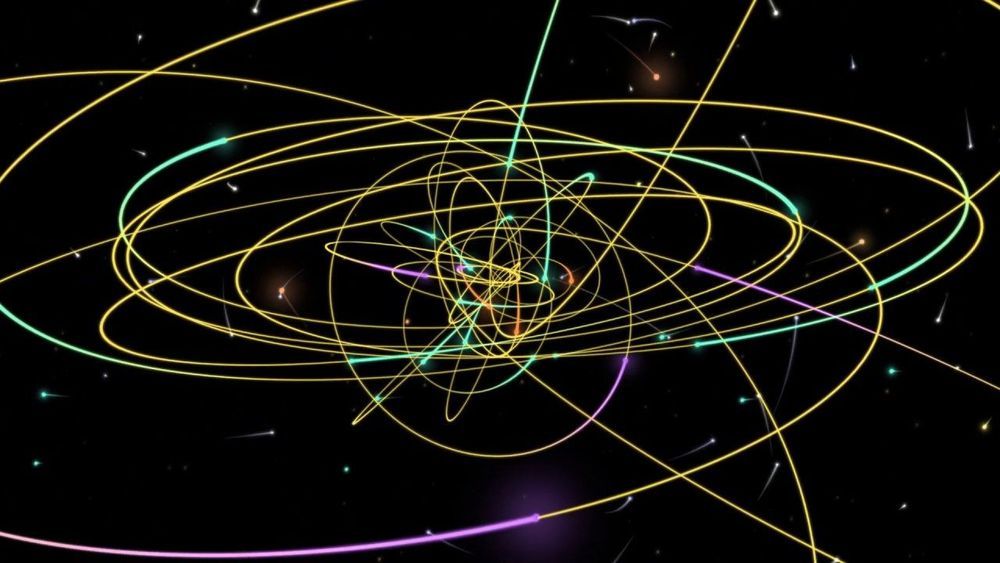
Astronomers have discovered a mysterious new class of objects at the heart of the Milky Way, unlike anything else found previously in our galaxy. The objects “look like gas but behave like stars,” according to senior researcher Andrea Ghez, as they start off small and compact but are stretched to a larger size when they approach the supermassive black hole in the center of the galaxy.
The researchers believe these objects could teach us about the evolution of stars and what happens to celestial bodies in environments of extreme gravity.
The puzzle began in 2005 when astronomers identified an object near the center of our galaxy called G1, which seemed to be orbiting around the supermassive black hole there in a strange way. In following years, five more objects numbered G2 to G6 were discovered. At first, these objects were thought to be clouds of gas. But one odd thing researchers noticed was that when the object G2 came very close to the event horizon of the black hole, it wasn’t torn apart in the way they would have expected. Instead, it initially stretched out, before rebounding back toward its original state.