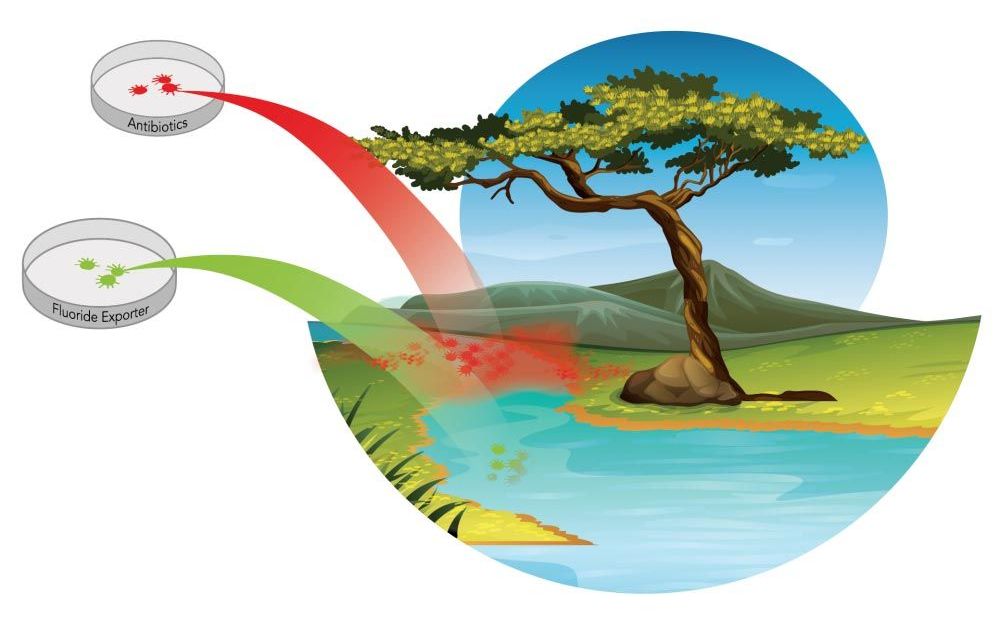
In Michelle O’Malley’s lab, a simple approach suggests a big leap forward in addressing the challenge of antibiotic-resistant bacteria.
Scientists have long been aware of the dangerous overuse of antibiotics and the increasing number of antibiotic-resistant microbes that have resulted. While over-prescription of antibiotics for medicinal use has unsettling implications for human health, so too does the increasing presence of antibiotics in the natural environment. The latter may stem from the improper disposal of medicines, but also from the biotechnology field, which has depended on antibiotics as a selection device in the lab.
“In biotech, we have for a long time relied on antibiotic and chemical selections to kill cells that we don’t want to grow,” said UC Santa Barbara chemical engineer Michelle O’Malley. “If we have a genetically engineered cell and want to get only that cell to grow among a population of cells, we give it an antibiotic resistance gene. The introduction of an antibiotic will kill all the cells that are not genetically engineered and allow only the ones we want — the genetically modified organisms [GMOs] — to survive. However, many organisms have evolved the means to get around our antibiotics, and they are a growing problem in both the biotech world and in the natural environment. The issue of antibiotic resistance is a grand challenge of our time, one that is only growing in its importance.”