Theoretical chemists at Princeton University have pioneered a strategy for modeling quantum friction, or how a particle’s environment drags on it, a vexing problem in quantum mechanics since the birth of the field. The study was published in the Journal of Physical Chemistry Letters (“Wigner–Lindblad Equations for Quantum Friction”). “It was truly a most challenging research project in terms of technical details and the need to draw upon new ideas,” said Denys Bondar, a research scholar in the Rabitz lab and corresponding author on the work.
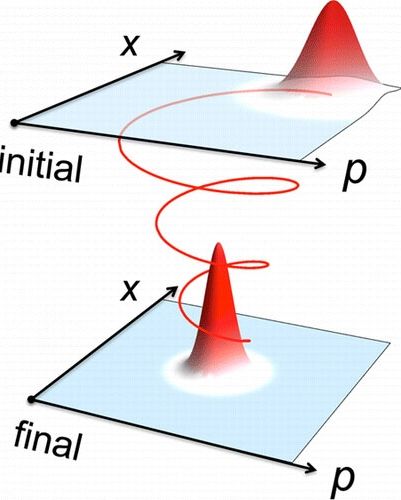
Researchers construct a quantum counterpart of classical friction, a velocity-dependent force acting against the direction of motion. In particular, a translationary invariant Lindblad equation is derived satisfying the appropriate dynamical relations for the coordinate and momentum (i.e., the Ehrenfest equations). Numerical simulations establish that the model approximately equilibrates. (© ACS)
Quantum friction may operate at the smallest scale, but its consequences can be observed in everyday life. For example, when fluorescent molecules are excited by light, it’s because of quantum friction that the atoms are returned to rest, releasing photons that we see as fluorescence. Realistically modeling this phenomenon has stumped scientists for almost a century and recently has gained even more attention due to its relevance to quantum computing.