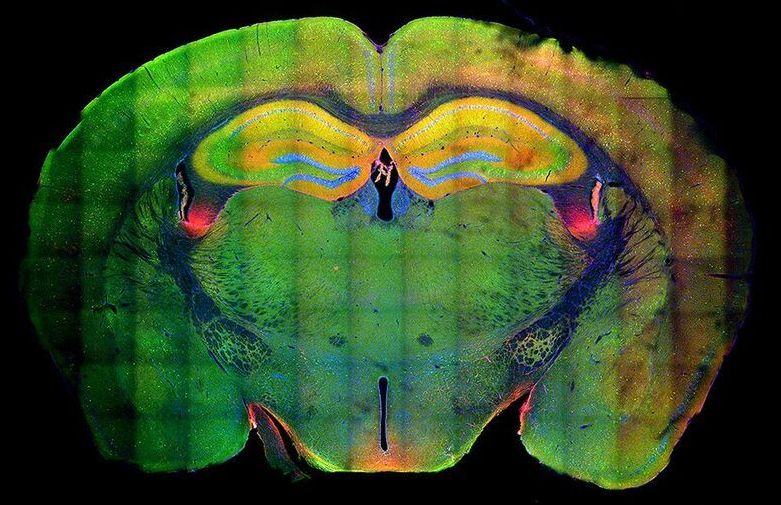
Researchers see structural changes in genetic material that allow memories to strengthen when remembered.
Category: biotech/medical
New semiconductor lasers work with small, portable coolers, enabling applications outside laboratories.
A biotech startup from Spain is making batteries that can power lights, music, and screens by using electrcity generated from soil microbes.
In a review published in the journal *Science*, Jain and Steele Laboratories colleagues Hadi T. Nia, PhD, and Lance L. Munn, PhD, describe four distinct physical hallmarks of cancer that affect both cancer cells and the tumor microenvironment, contributing to both tumor growth and the development of resistance to powerful cancer drugs.
One widely accepted model of cancer holds that a normal cell goes rogue because of genetic mutations or an environmental insult. In this model, the altered cell starts replicating out of control and takes over normal tissues, displaying eight hallmarks that include the ability to promote and sustain the growth of tumors, evade immune system attempts to suppress growth, stimulate blood flow to tumors and both invade local tissues and metastasize (spread) elsewhere in the body.
But this model fails to take into account how physical processes affect tumor progression and treatment, say the authors. In addition to the aforementioned eight biological hallmarks of cancer proposed by Robert Weinberg, PhD, from MIT, and Douglas Hanahan, PhD, from the Swiss Federal Institute of Technology in Lausanne, Jain and colleagues propose adding four distinct physical hallmarks that capture the biomechanical abnormalities in tumors: elevated solid stress; elevated interstitial fluid pressure; increased stiffness and altered material properties; and altered tissue micro-architecture.
Three decades of research in the Steele Laboratories led to the discovery and clinical translation of the first two hallmarks. “Solid stresses are created as proliferating and migrating cells push and stretch solid components of the surrounding tissue. They are large enough to compress blood and lymphatic vessels in and around tumors, impairing blood flow and the delivery of oxygen, drugs and immune cells,” Jain says.
Elevated interstitial fluid pressure is caused by abnormally permeable blood vessels in tumors leaking blood plasma into tissues surrounding the tumor, and by insufficient drainage of lymphatic fluid. The interstitial fluid carries various growth factors with it, causing edema (swelling), elution (release) of drugs and growth factors, and facilitating cancer invasion of local and distant tissues.
Increased stiffness is caused by the deposition of cellular matrix (scaffolding) and remodeling of tissues. This stiffness has traditionally been used as a diagnostic marker for tumor growth, and more recently it has come to be recognized as a marker for prognosis. Increased stiffness activates signaling pathways that promote proliferation, invasiveness and metastasis of cancer cells, Jain explains.
“Finally, when normal tissue architecture is disrupted by cancer growth and invasion, micro-architecture is altered,” he says. “Stromal (supporting) cells, cancer cells and extracellular matrix adopt new organization. This changes the interactions between an individual cell and its surrounding matrix and cells, which affects signaling pathways associated with invasion and metastasis.”
Jain says that with the review article in Science, he and his colleagues hope to bridge the gap between the physical and biological sciences “by exploring the biological origins and repercussions of the physical hallmarks of cancer from the perspectives of cancer biologists and oncologists — who work to understand and overcome the physical abnormalities at the bench and in the clinic — and from the perspectives of physicists and engineers who develop new models and strategies for research, diagnosis and treatment.”
An evolving understanding of cancer that incorporates the physical properties of tumors and their surrounding tissues into existing biologic and genetic models can direct cancer researchers down previously uncharted avenues, potentially leading to new drugs and new treatment strategies, say investigators from Massachusetts General Hospital (MGH), Harvard Medical School (HMS) and the Ludwig Center at HMS.
“We believe that progress in cancer research relies on close collaboration between cancer biologists, oncologists, physical scientists and engineers. A comprehensive understanding of the physical hallmarks of cancer requires a rigorous and broad perspective spanning the physical and biological sciences,” says Rakesh K. Jain, PhD, an investigator in the Edwin L. Steele Laboratories in the Department of Radiation Oncology at MGH and HMS.
In a review published in the journal Science, Jain and Steele Laboratories colleagues Hadi T. Nia, PhD, and Lance L. Munn, PhD, describe four distinct physical hallmarks of cancer that affect both cancer cells and the tumor microenvironment, contributing to both tumor growth and the development of resistance to powerful cancer drugs.
In this interview to Allison Duettmann, Carolina Reis, OneSkin’s CEO, describes the results of the prove of concept clinical study that the company performed for the product launched in the market some weeks ago, and explains more thoroughly the possible mechanisms of action involved in the reduction of senescent cells in the skin.
Zoom Transcription: https://otter.ai/s/DxPPE-AMSl6VdZa4K8dkDQ
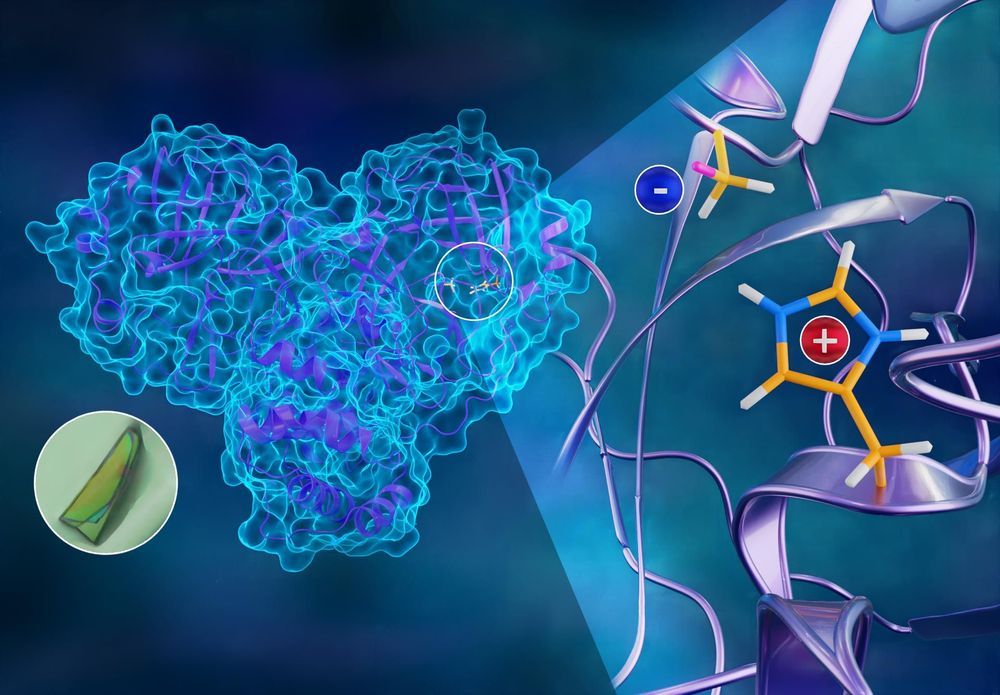
To better understand how the novel coronavirus behaves and how it can be stopped, scientists have completed a three-dimensional map that reveals the location of every atom in an enzyme molecule critical to SARS-CoV-2 reproduction.
Researchers at the Department of Energy’s Oak Ridge National Laboratory used neutron scattering to identify key information to improve the effectiveness of drug inhibitors designed to block the virus’s replication mechanism. The research is published in the Journal of Biological Chemistry.
The SARS-CoV-2 virus, which causes the COVID-19 disease, expresses long chains of proteins composed of approximately 1,900 amino acid residues. For the virus to reproduce, those chains have to be broken down and cut into smaller strands by an enzyme called the main protease. The active protease enzyme is formed from two identical protein molecules held together by hydrogen bonds. Developing a drug that inhibits or blocks the protease activity will prevent the virus from replicating and spreading to other cells in the body.
A closer look at Stentrode, the Brain Computer Interface that interacts with the brain via blood vessels. Recent paper demonstrating it working in 2 ALS patients.
Han from WrySci HX goes through the very interesting brain computer interface called Stentrode that can let you tweet with your mind. As a BCI, it’s a rival to Neuralink, Kernal, and Openwater. Find out about its background, how it works, why it’s the most unique BCI, and some results from its clinical trials. More below ↓↓↓
Subscribe! =]
Please consider supporting 🙏
Patreon: https://www.patreon.com/wrysci_hx
OnlyFans: https://onlyfans.com/han_xavier
Follow me on twitter: https://twitter.com/han_xavier_
Or better yet, consider supporting any of the following =].
Li’l Kurt is a genetic marvel.
In an effort to increase genetic diversity among horses, scientists have gone sci-fi and used frozen 40-year-old cells to create Kurt, the very first clone of a Przewalski’s horse.
🐴 You love badass animals. So do we. Let’s nerd out over them together.
Mice who ate a diet high in fat and cholesterol were more likely to see their hair turn from black to white and experience hair loss. The diet also appeared to cause inflammation of the skin.
In the first stage of the study, the researchers genetically modified mice to develop atherosclerosis, a condition in which fat deposits form in the arteries.
They then fed mice either a Western diet high in fat and cholesterol or untreated rat chow from the age of 12 to 20 weeks. As expected, the mice who consumed the Western diet saw their hair turn white and fall out, and develop skin lesions. And the longer the mice ate the diet, the worse their symptoms became. By week 36, three quarters of the animals had skin lesions.
New research[1] presented at the 29th EADV Congress, EADV Virtual, shows that socks coated in zinc oxide nanoparticles (ZnO-NPs) can prevent bromodosis (foot odor) and pitted keratolysis (bacterial infection causing smelly feet), reducing the negative impact this embarrassing condition has on quality of life.[2]
Developed by the Royal Thai Airforce, the ZnO-NP-coated socks were trialed in a real-life setting by researchers at Siriraj Hospital, Mahidol University in Thailand. They found that the antibacterial efficacy of ZnO-NPs, along with its safety and compatibility with human skin, makes it the perfect compound to incorporate into textiles, including socks, to prevent unpleasant foot odor.
The double-blinded, randomized, controlled trial was conducted with 148 cadets at the Thai Naval Rating School. Bromodosis and pitted keratolysis are a common complaint in military personnel, with foot lesions, including pitted keratosis, occurring in over a third of naval cadets in Thailand (38.5%).[2]