Hype aside demonstration that epigentic reprogramming can reverse some of the aging process is an important step forward for progress. We can expect to see this moving to human trials in the next decade or so making the future an exciting possibility.
Science is increasingly coming to the conclussion that aging is amenable to intervention and that it is a plastic process that we can manipulate. More research in this week shows that aging is indeed elastic and is not a one way process at all. The sooner society accepts what the data from the labs is showing the sooner we can cure age-related diseases for healthier longer lives!
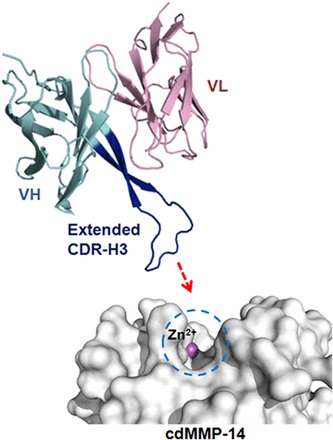
“We did not correct the mutation that causes premature aging in these mice,” lead researcher Juan Carlos Izpisua Belmonte said in a recent statement. “We altered aging by changing the epigenome, suggesting that aging is a plastic process.”