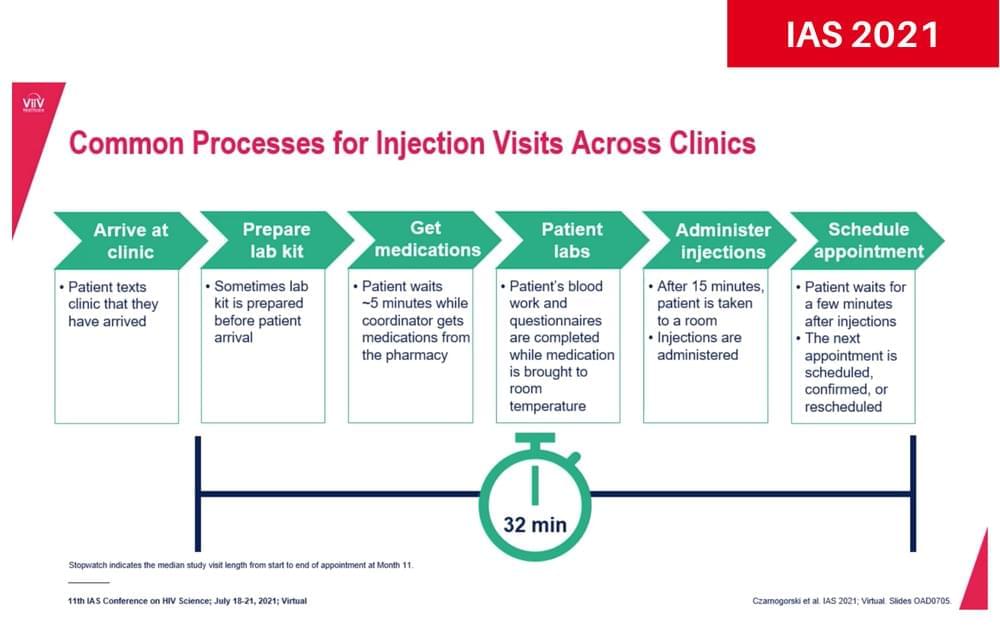
The long-acting injectable HIV medications cabotegravir and rilpivirine, which are administered by a healthcare provider once a month, can be successfully implemented in health practises in the United States, according to a study presented at the 11th International AIDS Society Conference on HIV Science (IAS 2021). What’s more, providers and people with HIV encountered few barriers to giving or receiving the injections despite changes in health services during COVID-19.
“Over the course of a year, even with the added challenges of COVID-19, the barriers that providers and patients thought they would face turned out not to be as concerning as originally thought,” Dr Maggie Czarnogorski of ViiV Healthcare said in a press release.
In October 2020, the European Medicines Agency (EMA) approved the injectable combination regimen, which consists of an extended-release formulation of ViiV Healthcare’s new integrase inhibitor cabotegravir (Vocabria) plus an injectable version of Janssen’s non-nucleoside reverse transcriptase inhibitor rilpivirine (Rekambys, sold in pill form as Edurant). The US Food and Drug Administration (FDA) approved the combination, sold in North America as Cabenuva, in January 2021.