Solar cells based on perovskite compounds could soon make electricity generation from sunlight even more efficient and cheaper. The laboratory efficiency of these perovskite solar cells already exceeds that of the well-known silicon solar cells. An international team led by Stefan Weber from the Max Planck Institute for Polymer Research in Mainz has found microscopic structures in perovskite crystals that can guide the charge transport in the solar cell. Clever alignment of these electron highways could make perovskite solar cells even more powerful.
When solar cells convert sunlight into electricity, the electrons of the material inside the cell absorb the energy of the light. Traditionally, this light-absorbing material is silicon, but perovskites could prove to be a cheaper alternative. The electrons excited by the sunlight are collected by special contacts on the top and bottom of the cell. However, if the electrons remain in the material for too long, they can lose their energy again. To minimize losses, they should therefore reach the contacts as quickly as possible.
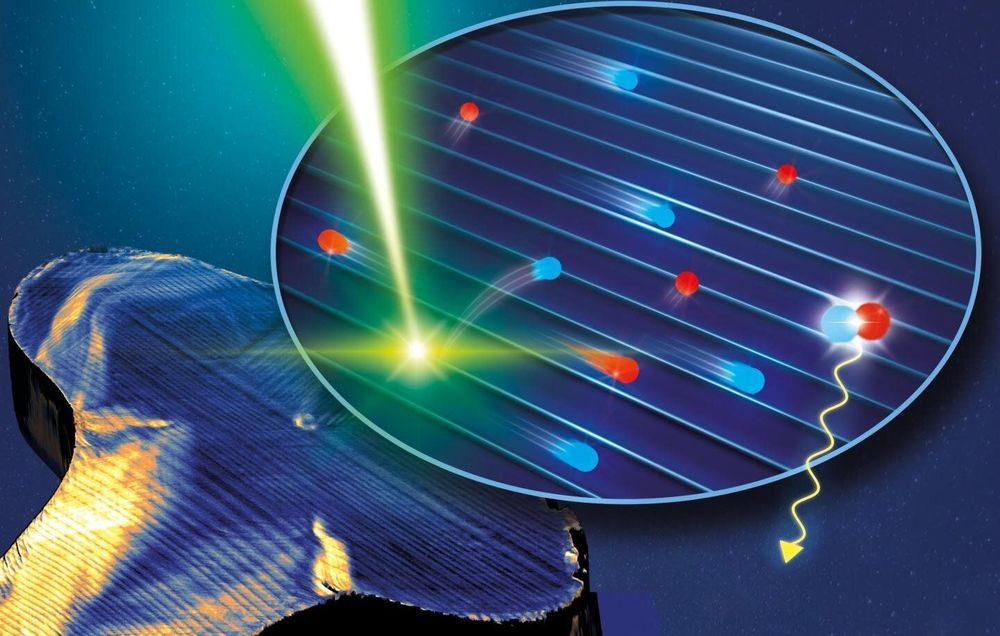
Microscopically small structures in the perovskites—so-called ferroelastic twin domains—could be helpful in this respect: They can influence how fast the electrons move. An international research group led by Stefan Weber at the Max Planck Institute for Polymer Research in Mainz discovered this phenomenon. The stripe-shaped structures that the scientists investigated form spontaneously during the fabrication of the perovskite by mechanical stress in the material. By combining two microscopy methods, the researchers were able to show that electrons move much faster parallel to the stripes than perpendicular to them. “The domains act as tiny highways for electrons,” compares Stefan Weber.