Rice University researchers have discovered a hidden symmetry in the chemical kinetic equations scientists have long used to model and study many of the chemical processes essential for life.
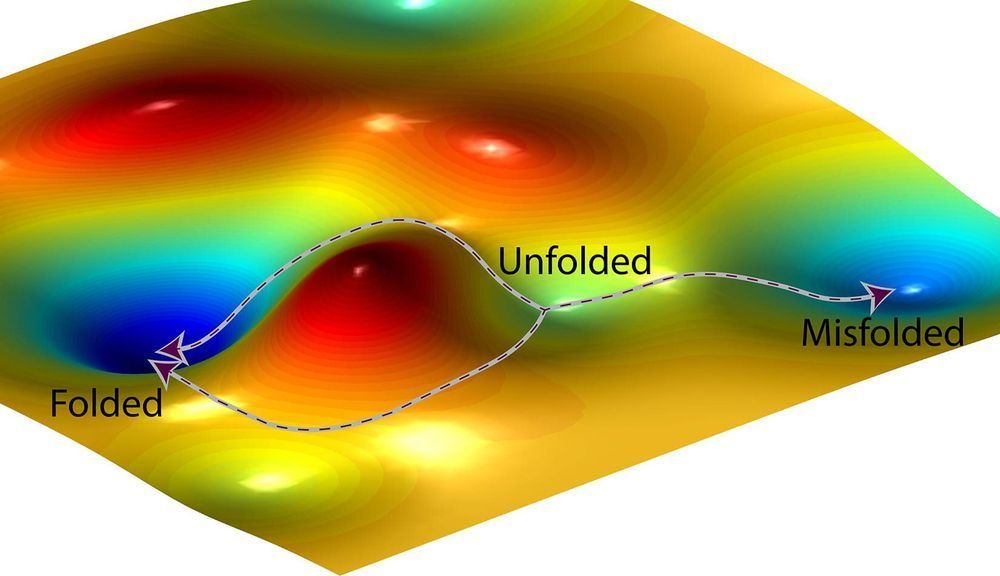
The find has implications for drug design, genetics and biomedical research and is described in a study published this month in the Proceedings of the National Academy of Sciences. To illustrate the biological ramifications, study co-authors Oleg Igoshin, Anatoly Kolomeisky and Joel Mallory of Rice’s Center for Theoretical Biological Physics (CTBP) used three wide-ranging examples: protein folding, enzyme catalysis and motor protein efficiency.
In each case, the researchers demonstrated that a simple mathematical ratio shows that the likelihood of errors is controlled by kinetics rather than thermodynamics.