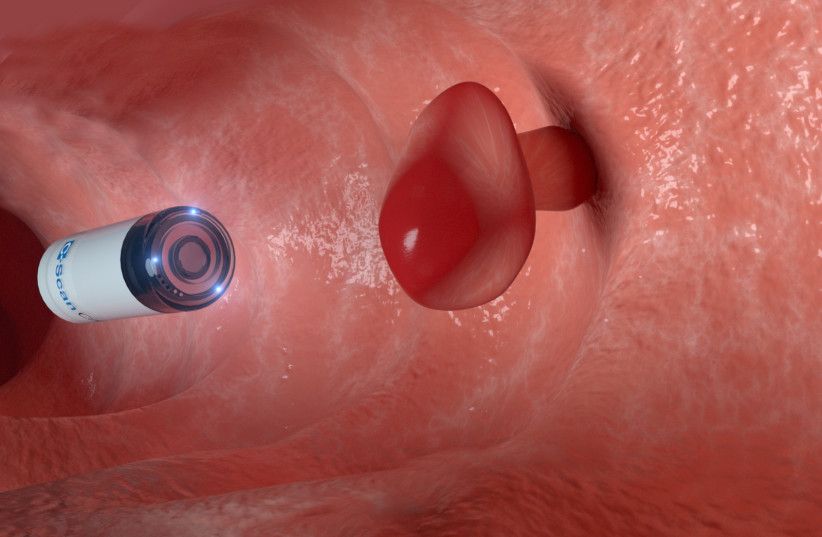
A swallowable capsule that can identify warning signs of colorectal cancer is moving closer to the American market, promising an Israeli-led revolution in colorectal cancer prevention.
“When we ask patients and physicians, we get a clear answer that the device has the potential to change the natural history of colon cancer screening,” said Ovadia. “Since the device is safe, not an intervention and there is no need for preparation, we have resolved most of the barriers preventing any patient of the recommended age from undergoing screening. There is no reason now for a patient not to perform the study.”
According to Prof. Nadir Arber, the principal investigator for C-Scan clinical trials and the head of the Health Promotion Center and Integrated Cancer Prevention Center at Tel Aviv Sourasky Medical Center, the C-Scan system “can change the landscape of colorectal cancer prevention worldwide.”
“Although colorectal cancer can be prevented through the detection of precancerous polyps, screening adherence remains low due to the bowel preparation, sedation and invasiveness associated with current screening methods, and Gastroenterologists certainly struggle with that,” Arber told the Post.