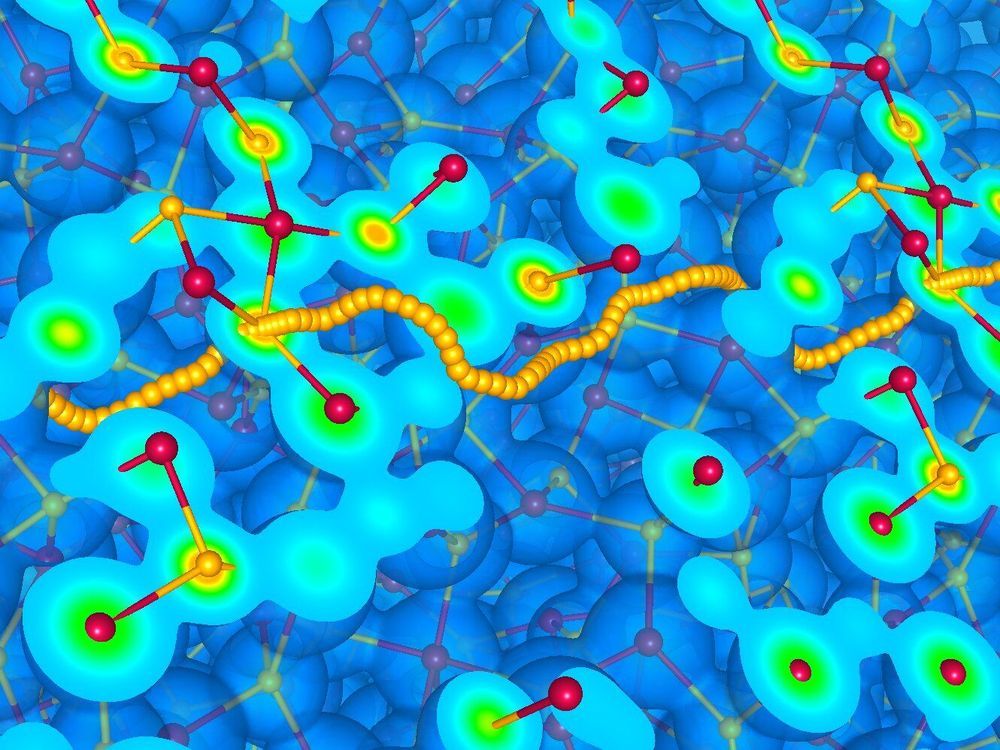
Oxidation numbers have so far eluded any rigorous quantum mechanical definition. A new SISSA study, published in Nature Physics, provides such a definition based on the theory of topological quantum numbers, which was honored with the 2016 Nobel Prize in Physics, awarded to Thouless, Haldane and Kosterlitz. This result, combined with recent advances in the theory of transport achieved at SISSA, paves the way to an accurate, yet tractable, numerical simulation of a broad class of materials that are important in energy-related technologies and planetary sciences.
Every undergraduate student in the natural sciences learns how to associate an integer oxidation number to a chemical species participating in a reaction. Unfortunately, the very concept of oxidation state has thus far eluded a rigorous quantum mechanical definition, so that no method was known until now to compute oxidation numbers from the fundamental laws of nature, let alone demonstrate that their use in the simulation of charge transport does not spoil the quality of numerical simulations. At the same time, the evaluation of electric currents in ionic conductors, which is required to model their transport properties, is presently based on a cumbersome quantum-mechanical approach that severely limits the feasibility of large-scale computer simulations. Scientists have lately noticed that a simplified model where each atom carries a charge equal to its oxidation number may give results in surprising good agreement with rigorous but much more expensive approaches.