Awesome!
What if industrial waste water could become fuel? With affordable, long-lasting catalysts, water could be split to produce hydrogen that could be used to power fuel cells or combustion engines.
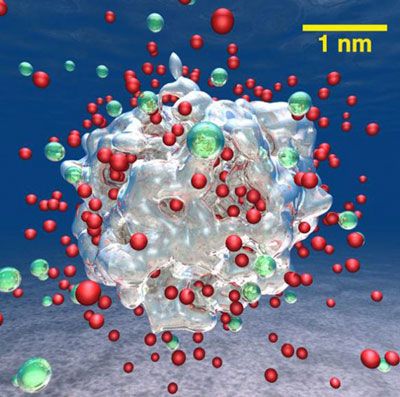
By conducting complex simulations, scientists showed that adding lithium to aluminum nanoparticles results in orders-of-magnitude faster water-splitting reactions and higher hydrogen production rates compared to pure aluminum nanoparticles. The lithium allowed all the aluminum atoms to react, which increased yields (Nano Letters, “Hydrogen-on-demand using metallic alloy nanoparticles in water”).
A snapshot from a large quantum molecular dynamics simulation of the production of hydrogen molecules (green) from an aluminum-lithium alloy nanoparticle containing 16,661 atoms (represented by the silver contour of charge density) and dissolved charged lithium atoms (red). For clarity, the water molecules were removed from the snapshot. Simulations were carried out at the Argonne Leadership Computing Facility.