Tokamak Energy of the UK has built the ST40 prototype fusion reactor and they aim to reach 100 million degrees celsius by the end of 2018. They have already reached 15 million degrees.



You can generate electricity from oil, you can produce it from natural gas, you can make it from nuclear energy, and you can channel it from the sun, via solar energy conversion systems. You can even generate electricity from photosynthetic bacteria, also known as cyanobacteria, based on a new innovation developed at the Technion. As published in a study in the journal, Nature Communications, the Technion researchers have developed an energy-producing system that exploits both the photosynthesis and respiratory processes that cyanobacteria undergo, with the harvested energy leveraged to generate electricity based on hydrogen.
The study was conducted by three Technion faculty members: Professor Noam Adir from the Schulich Faculty of Chemistry, Professor Gadi Schuster from the Faculty of Biology, and Professor Avner Rothschild, from the Faculty of Materials Science and Engineering. The work involved collaboration between Dr. Gadiel Saper and Dr. Dan Kallmann, as well as colleagues from Bochum, Germany and the Weizmann Institute of Science. It was supported by various bodies, including the Nancy and Stephen Grand Technion Energy Program (GTEP), the Russell Berrie Nanotechnology Institute (RBNI), the Technion Hydrogen Technologies Research Lab (HTRL), the Adelis Foundation, the Planning and Budgeting Committee’s I-CORE program, the Israel Science Foundation, the USA-Israel Binational Science Fund (BSF) and the German research fund (DFG-DIP).
Scientists have long considered cyanobacteria a possible energy source. Cyanobacteria belong to a family of bacteria common to lakes, seas, and many other habitats. The bacteria use photosynthetic mechanisms that enable them to generate energy from sunlight. They also generate energy in the dark, via respiratory mechanisms based on digestion and degradation of sugar.



Are we ready?
Batteries powered by radioactive materials have been around for more than a century, but what they promise in power they usually lose in bulk.
Not so with a new kind of power source, which combines a novel structure with a nickel isotope to pack ten times more power than an electrochemical cell of the same size. The only question is, are we ready to go nuclear?
A team of Russian researchers have put a new spin on technology that uses the beta decay of a radioactive element to create differences in voltage.
James Woodward and the Space Studies Institute has a Phase 2 NASA Innovative Advanced funded study. They are looking at the implementation of an innovative thrust producing technology for use in NASA missions involving in space main propulsion.
Dr. Heidi Fearn explained in a video made in 2017 how just scaling the power and size of the Mach effect propulsion causes problems. (heat, arcing and other problems). They currently believe they can scale the device to one newton of propulsion and then create large arrays of the devices for more thrust. The constant thrust could last for years or decades by using a nuclear power source.
For Mach effect propellantless propulsion it will be better to go to an array of smaller devices.
One of the possible pathways to limitless and clean energy can be found in hollow, doughnut-shaped chambers known as tokamak nuclear fusion reactors. A relatively new player on the scene, a UK company called Tokamak Energy, is claiming a new milestone in the area after heating its ST40 device to 15 million degrees Celsius, similar to temperatures found at the center of the Sun.
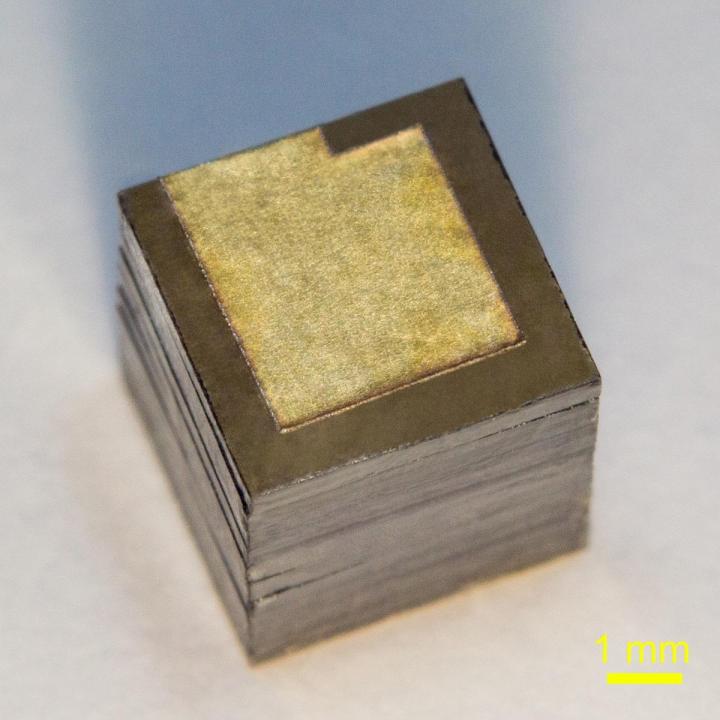
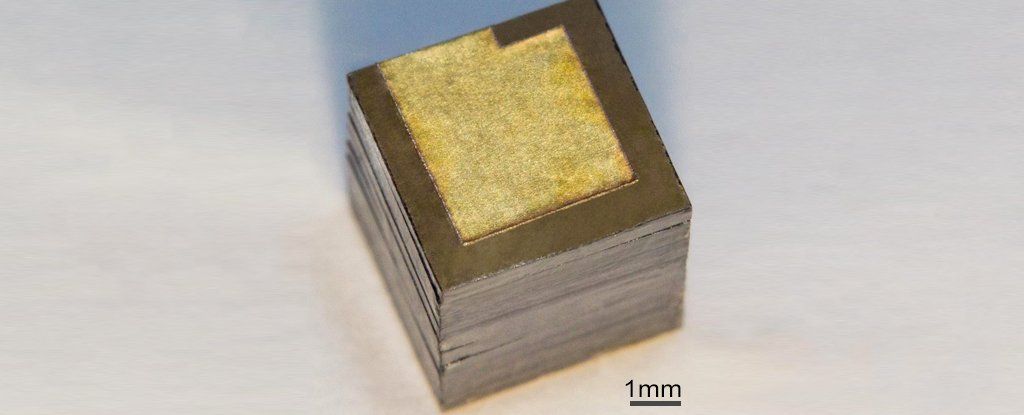
Russian researchers from the Moscow Institute of Physics and Technology (MIPT), the Technological Institute for Superhard and Novel Carbon Materials (TISNCM), and the National University of Science and Technology MISIS have optimized the design of a nuclear battery generating power from the beta decay of nickel-63, a radioactive isotope. Their new battery prototype packs about 3,300 milliwatt-hours of energy per gram, which is more than in any other nuclear battery based on nickel-63, and 10 times more than the specific energy of commercial chemical cells. The paperwas published in the journal Diamond and Related Materials.
Conventional batteries
Ordinary batteries powering clocks, flashlights, toys, and other compact autonomous electrical devices use the energy of so-called redox chemical reactions. In them, electrons are transferred from one electrode to another via an electrolyte. This gives rise to a potential difference between the electrodes. If the two battery terminals are then connected by a conductor, electrons start flowing to remove the potential difference, generating an electric current. Chemical batteries, also known as galvanic cells, are characterized by a high power density — that is, the ratio between the power of the generated current and the volume of the battery. However, chemical cells discharge in a relatively short time, limiting their applications in autonomous devices. Some of these batteries, called accumulators, are rechargeable, but even they need to be replaced for charging. This may be dangerous, as in the case of a cardiac pacemaker, or even impossible, if the battery is powering a spacecraft.