By altering the NOVA1 gene, researchers were able to “Neanderthal-izes” a brain organoid model. Study reveals there is only a one gene difference between the modern human brain and that of our extinct ancestors.


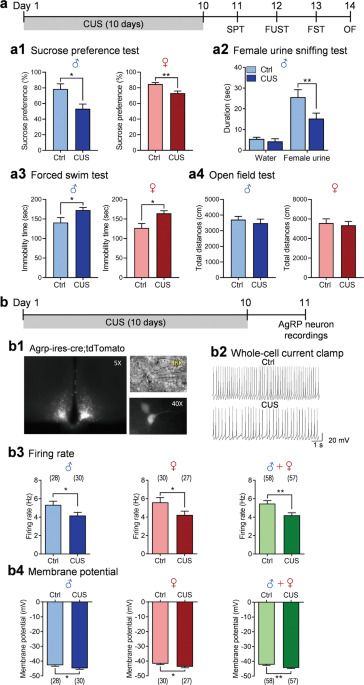
Previous studies have shown that AgRP neurons in the arcuate nucleus (ARC) respond to energy deficits and play a key role in the control of feeding behavior and metabolism. Here, we demonstrate that chronic unpredictable stress, an animal model of depression, decreases spontaneous firing rates, increases firing irregularity and alters the firing properties of AgRP neurons in both male and female mice. These changes are associated with enhanced inhibitory synaptic transmission and reduced intrinsic neuronal excitability. Chemogenetic inhibition of AgRP neurons increases susceptibility to subthreshold unpredictable stress. Conversely, chemogenetic activation of AgRP neurons completely reverses anhedonic and despair behaviors induced by chronic unpredictable stress.

What if our bodies kept evolving? And are there body parts that will disappear one day?
The First 1000 people to click this link get a FREE SKILLSHARE PREMIUM MEMBERSHIP: https://skl.sh/asapscience01211
Our backs hurt, ankles break and feet are busted! Not to mention having a baby is dangerous and our eyes are built backwards. There is a lot that doesn’t work in our bodies, so today we are going to explain the perfectly evolved human. Evolutionary biologists have been battling this scenario for years so we explain it all. Including the need for ostrich feet, bipedal bodies, bilateral symmetry, rewiring neurons in the eye and having dog ears! Let us know if you would want this body!?
References:
Metazoa: Animal Life and the Birth of the Mind — by Peter Godfrey Smith.
https://leakeyfoundation.org/2015why-walk-on-two-legs/#:~:text=The%20toes%20are%20reduced%20in, stable%2C%20rigid%20base%20for%20propulsion.
https://www.earthdate.org/node/131
https://pubmed.ncbi.nlm.nih.gov/30772945/
https://pubmed.ncbi.nlm.nih.gov/31163155/
https://pubmed.ncbi.nlm.nih.gov/30482358/
https://pubmed.ncbi.nlm.nih.gov/29787621/
https://pubmed.ncbi.nlm.nih.gov/28406563/

Cell-Intrinsic Learning And Memory Storage Dynamics — Dr. David Glanzman Ph.D., Professor, in the Department Integrative Biology and Physiology, at UCLA College of the Life Sciences.
Dr. David Glanzman is Professor, in the Department Integrative Biology and Physiology, at UCLA College of the Life Sciences, Professor in the Department of Neurobiology in the David Geffen School of Medicine, and Member, Brain Research Institute.
Dr. Glanzman has a B.A. in Psychology from Indiana University Bloomington and a Ph.D. in Psychology from Stanford University.
Dr. Glanzman is interested in the cell biology of learning and memory in simple organisms.
In Dr. Glanzman’s lab research they use two animals, the marine snail Aplysia californica, and the zebrafish (Danio rerio).
Dr. Glanzman’s work on Aplysia: This invertebrate has a comparatively simple nervous system (~ 20000 neurons) that provides a valuable experimental model for understanding the cellular mechanisms that underlie simple forms of learning, such as habituation, sensitization, and classical conditioning.
Another experimental advantage of Aplysia is that sensory and motor neurons that mediate specific reflexes of the animal can be placed into dissociated cell culture where they will reform their synaptic connections.
These in vitro sensorimotor synapses are extremely useful for cellular and molecular studies of short-and long-term learning-related synaptic plasticity.
After tracing the origins of schizophrenia to genes expressed in the placenta while in utero, scientists have now zeroed in on the combination of risk factors that could predict which infants are at greatest risk of developing the condition later in life.
The findings reinforce an emerging picture of schizophrenia as a genetic disorder, with a fate determined by complications that can arise during pregnancy.
Researchers from the Lieber Institute for Brain Development at Johns Hopkins University and the University of North Carolina in the US analysed the relationship between key genes and cognitive development in the first few years after birth.
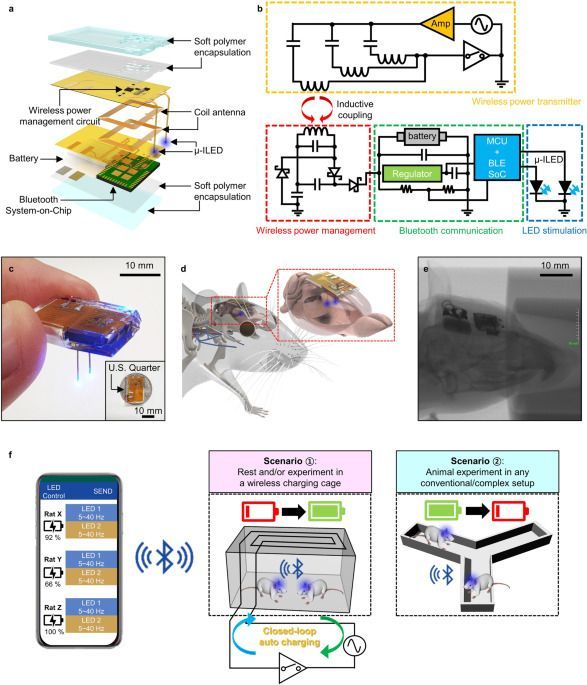
Although wireless optogenetic technologies enable brain circuit investigation in freely moving animals, existing devices have limited their full potential, requiring special power setups. Here, the authors report fully implantable optogenetic systems that allow intervention-free wireless charging and controls for operation in any environment.
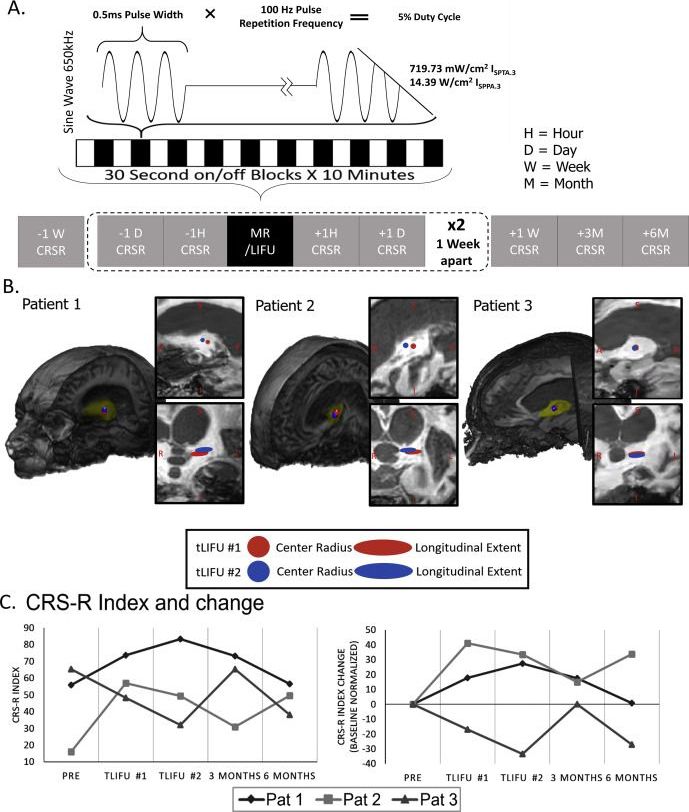
Despite great advances in the field of intensive care, when patients survive a severe.
Brain injury but remain in a chronic Vegetative State (VS) or Minimally Conscious.
State (MCS) (i.e., a disorder of consciousness; DOC), little can be done to promote.
Recovery [1]. To date, several restorative strategies have been investigated in DOC.