Scientists have known for decades that aerobic exercise strengthens the brain and contributes to the growth of new neurons, but few studies have examined how yoga affects the brain. A review of the science finds evidence that yoga enhances many of the same brain structures and functions that benefit from aerobic exercise.
The review, published in the journal Brain Plasticity, focused on 11 studies of the relationship between yoga practice and brain health. Five of the studies engaged individuals with no background in yoga practice in one or more yoga sessions per week over a period of 10–24 weeks, comparing brain health at the beginning and end of the intervention. The other studies measured brain differences between individuals who regularly practice yoga and those who don’t.
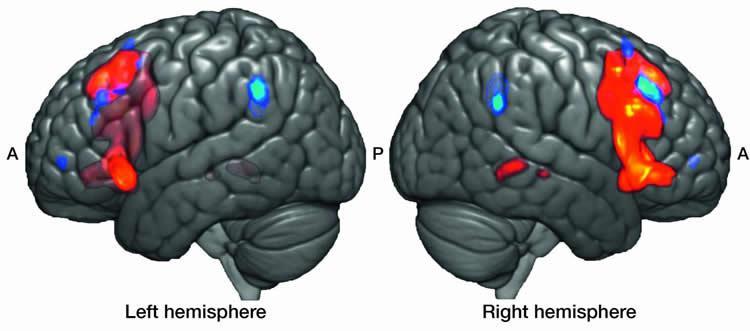
Each of the studies used brain-imaging techniques such as MRI, functional MRI or single-photon emission computerized tomography. All involved Hatha yoga, which includes body movements, meditation and breathing exercises.