Alpha-ketoglutarate could be safer than other potential anti-aging treatments.


Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.

Middle-aged mice that had the naturally-occurring metabolite alpha-ketaglutarate (AKG) added to their chow had a better “old age.” They were healthier as they aged and experienced a dramatically shorter time of disease and disability before they died, a first for research involving mammals. Results from the double-blinded study, published in Cell Metabolism, were based on clinically-relevant markers of healthspan.
Previous studies show that blood plasma levels of AKG can drop up to 10-fold as we age. Fasting and exercise, already shown to promote longevity, increase the production of AKG is not found in the normal diet, making supplementation the only feasible way to restore its levels.
“The standard for efficacy in research on aging is whether interventions actually improve healthspan. We’ve reached that mark here with a compound that is naturally produced by the body and is generally shown to be safe,” said Buck professor and senior author Gordon Lithgow, Ph.D… Noting that some of the mice did experience moderate lifespan extension (the average was around 12%), measures of healthspan increased more than 40 percent. Lithgow says the goal is always to compress the time of disease and frailty. “The nightmare scenario has always been life extension with no reduction in disability. In this study, the treated middle-aged mice got healthier over time. Even the mice that died early saw improvements in their health, which was really surprising and encouraging.”

Excerpts of talks and interviews on biological radical life extension given by some of the world top longevity scientists.
The compendium includes thoughts, predictions and claims made by the following longevity leaders (listed in alphabetical order):
Aubrey de Grey, PhD: https://en.wikipedia.org/wiki/Aubrey_de_Grey
David Sinclair, PhD: https://en.wikipedia.org/wiki/David_Andrew_Sinclair
George Church, PhD: https://en.wikipedia.org/wiki/George_Church_(geneticist)
Juan Carlos Izpisúa Belmonte, PhD: https://en.wikipedia.org/wiki/Juan_Carlos_Izpisua_Belmonte
María Blasco Marhuenda, PhD: https://en.wikipedia.org/wiki/Mar%C3%ADa_Blasco_Marhuenda
I added embedded subtitles in English when scientists speak in Spanish.
For subtitles in Spanish when scientists speak in English, just choose the option in Youtube to add the subtitles in Spanish I created.
These are some of my social media channels, you’re invited to keep in touch through any of them:
LinkedIn: https://www.linkedin.com/in/andresgrases/
Facebook: https://www.facebook.com/andres.grases
Instagram: https://www.instagram.com/andgrabri/
Youtube: https://www.youtube.com/andresgrases
This is my own website which includes a digital library with more than 26.000 links and growing, organized in 19 main categories and many other sub-categories: https://transhumanplus.com/
Ahhh, and if you haven’t done so, please consider subscribing to my YouTube channel smile

A striking new study has found young cancer survivors show high expression of a gene known to be an effective marker of aging. The researchers suggest this genetic biomarker could be used to identify cancer survivors most at risk of later-life frailty due to their treatment.
As we age, concentrations of a gene called p16INK4a gradually increase in our cells, making it a potentially useful molecular marker for aging. One of the gene’s roles is to slow cell division and reduce the proliferation of stem cells.
In a new study researchers set out to investigate p16INK4a levels in pediatric and young adult cancer survivors. The hypothesis was that increased p16INK4a levels could be an effective sign of frailty among young cancer survivors.

This is an excerpt of a conversation between Dr. Daniel Stickler and Brian Rose.
Dr. Stickler is the Medical Director for the Neurohacker Collective, a consultant for Google on epigenetics and AI in healthcare, and a lecturer at Stanford University.
Brian Rose is the founder of London Real, a curator of people worth watching. Its mission is to promote personal transformation through inspiration, self-discovery and empowerment.
CUENTA CON SUBTÍTULOS EN ESPAÑOL
To watch the entire conversation clic here: https://youtu.be/ynbaJ2038K0
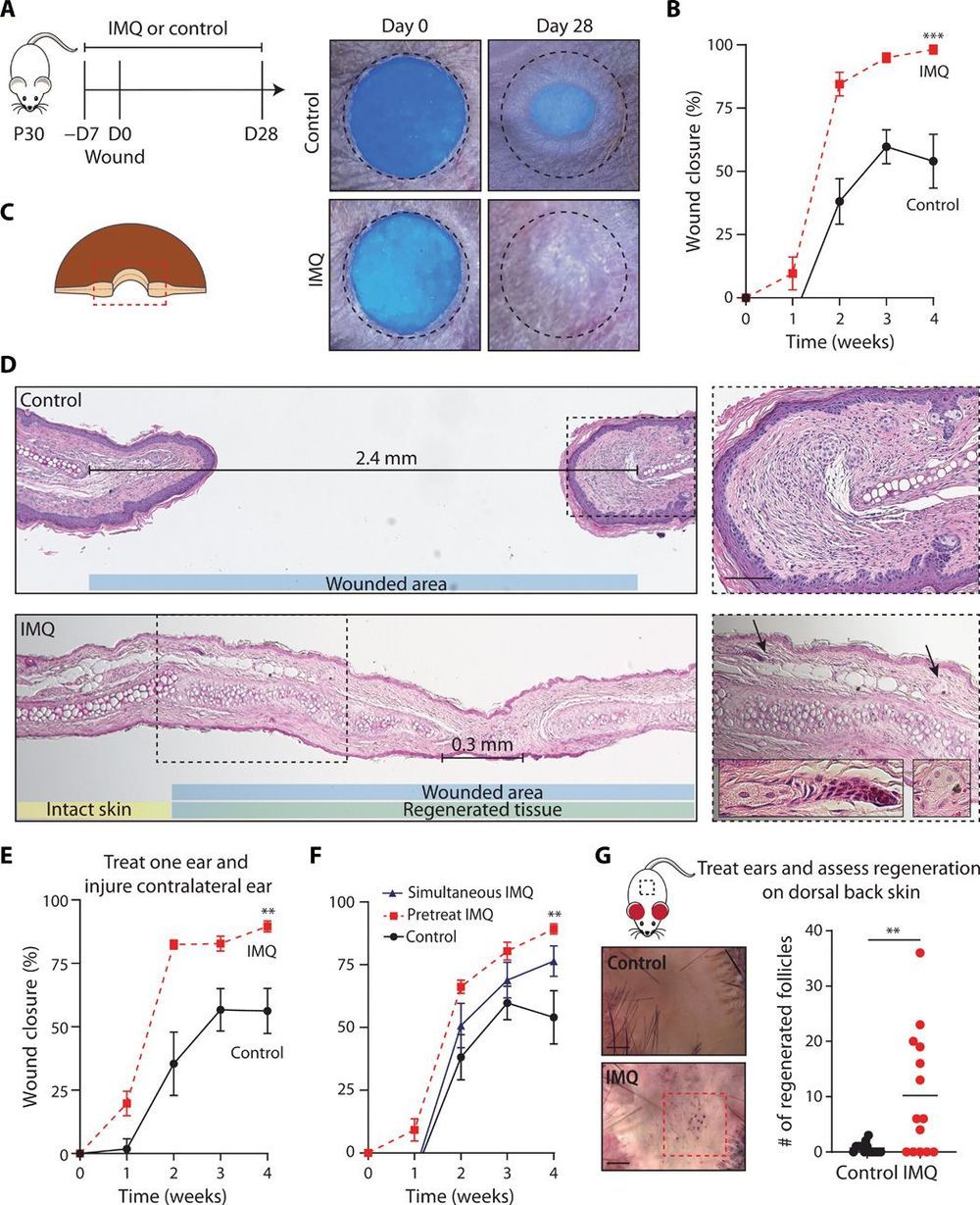
Could speed up healing.
Wound healing in mammalian skin often results in fibrotic scars, and the mechanisms by which original nonfibrotic tissue architecture can be restored are not well understood. Here, Wei et al. have shown that pharmacological activation of the nociceptor TRPA1, which is found on cutaneous sensory neurons, can limit scar formation and promote tissue regeneration. They confirmed the efficacy of TRPA1 activation in three different skin wounding mouse models, and they also observed that localized activation could generate a response at distal wound sites. TRPA1 activation induced IL-23 production by dermal dendritic cells, which activated IL-17–producing γδ T cells and promoted tissue regeneration. These findings provide insight into neuroimmune signaling pathways in the skin that are critical to mammalian tissue regeneration.
Adult mammalian wounds, with rare exception, heal with fibrotic scars that severely disrupt tissue architecture and function. Regenerative medicine seeks methods to avoid scar formation and restore the original tissue structures. We show in three adult mouse models that pharmacologic activation of the nociceptor TRPA1 on cutaneous sensory neurons reduces scar formation and can also promote tissue regeneration. Local activation of TRPA1 induces tissue regeneration on distant untreated areas of injury, demonstrating a systemic effect. Activated TRPA1 stimulates local production of interleukin-23 (IL-23) by dermal dendritic cells, leading to activation of circulating dermal IL-17–producing γδ T cells. Genetic ablation of TRPA1, IL-23, dermal dendritic cells, or γδ T cells prevents TRPA1-mediated tissue regeneration.
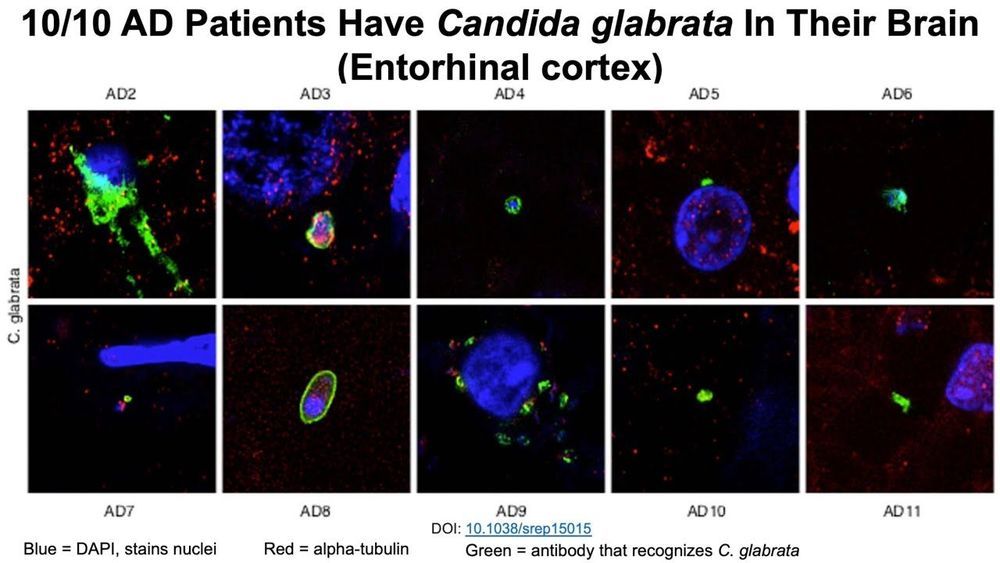
Here’s my latest video!
The incidence of fungi bloodstream infections increases during aging-is that a potential explanation for the presence of fungi in the brains of Alzheimer’s disease patients? Rapamycin is a known antifungal-is it effective against fungi that are found in the blood and brain?
Very interesting.
Despite the limited supply of organs available for patients on waitlists for transplantation, organs from older, deceased donors are frequently discarded or not utilized. Available older organs have the potential to close the gap between demand and supply that is responsible for the very long wait-times that lead to many patients not surviving the time it takes for an organ to become available. Older organs can also often provoke a stronger immune response and may put patients at greater risk of adverse outcomes and transplant rejection. But, as the world population ages, organs from older, deceased donors represent an untapped and growing resource for patients in need. Investigators from Brigham and Women’s Hospital are leading efforts to breathe new life into older organs by leveraging a new class of drugs known as senolytics, which target and eliminate old cells. Using clinical and experimental studies, the team presents evidence that senolytic drugs may help rejuvenate older organs, which could lead to better outcomes and a wider pool of organs eligible for donation. Results are published in Nature Communications.
“Older organs are available and have the potential to contribute to mitigating the current demand for organ transplantation,” said corresponding author Stefan G. Tullius, MD, Ph.D., chief of the Division of Transplant Surgery at the Brigham. “If we can utilize older organs in a safe way with outcomes that are comparable, we will take a substantial step forward for helping patients.”
As organs age, senescent cells accumulate. These cells, which no longer divide, escape the body’s usual means of destroying older, unneeded cells. Senescent cells release cell-free mitochondrial DNA (mt-DNA), which also accumulates in older organs. Recent studies have suggested that this rise in mt-DNA is tied to organ rejection.

Research looking at a possible new therapeutic approach for Alzheimer’s disease was recently published in the Journal of Neuroinflammation. The paper out of the University of Kentucky’s Sanders-Brown Center on Aging (SBCoA) is titled “Therapeutic Trem2 activation ameliorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition”. The work looked at targeting inflammation by using an antibody. Alzheimer’s disease and related dementias have no disease-modifying treatments at this time and represent a looming public health crisis given the continually growing aging population.
The paper explains that current therapeutic approaches to the treatment of Alzheimer’s disease focus on the major pathological hallmarks of the disease which are amyloid plaques and neurofibrillary tangles. They are the requirements for a diagnosis of Alzheimer’s disease. However, the authors say there has been an explosion of genetic data suggesting the risk for sporadic Alzheimer’s disease is driven by several other factors including neuroinflammation, membrane turnover and storage, and lipid metabolism.
In this study the researchers focused on triggering receptor expressed on myeloid cell-2 (TREM2). “TREM2 was identified several years ago as a gene that, when there’s a mutation, significantly increases risk of Alzheimer’s disease. The field thinks that this mutation reduces the function of the receptor, so we hypothesized that targeting TREM2 to increase its function might be a valid treatment for Alzheimer’s,” explained Donna Wilcock, SBCoA associate director.