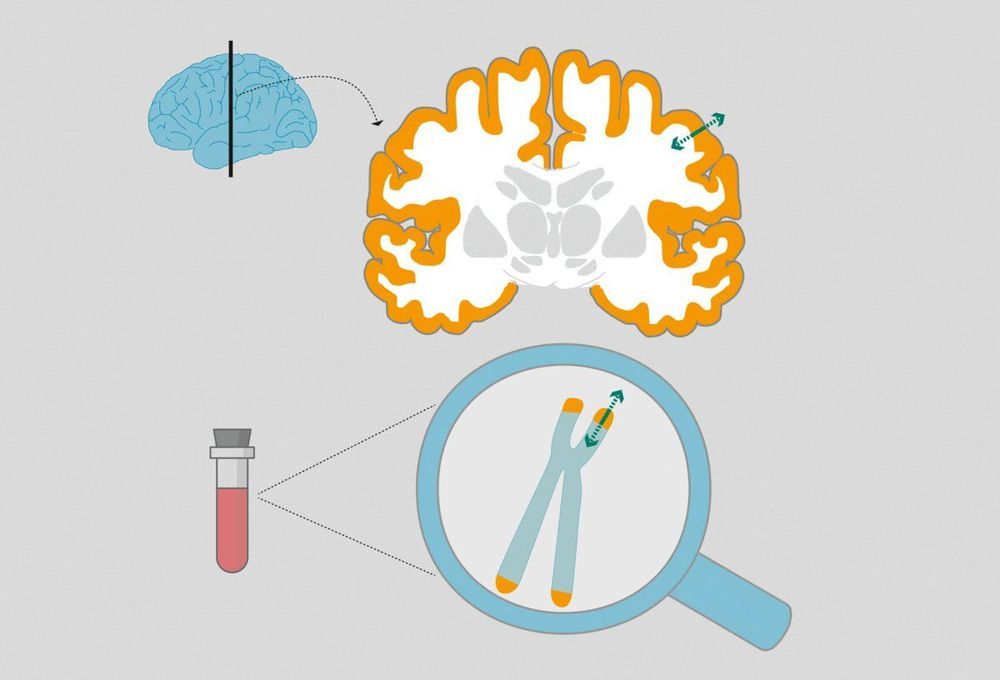
Telomeres are the protective caps of our chromosomes and play a central role in the aging process. Shorter telomeres are associated with chronic diseases and high stress levels can contribute to their shortening. A new study now shows that if telomeres change in their length, that change is also reflected in our brain structure. This association was identified by a team of scientists including Lara Puhlmann and Pascal Vrtička from the Max Planck Institute for Cognitive Brain Sciences in Leipzig together with Elissa Epel from the University of California and Tania Singer from the Social Neuroscience Lab in Berlin as part of Singer’s ReSource Project.
Telomeres are protective caps at the ends of chromosomes that become shorter with each cell division. If they become so short that the genes they protect could be damaged, the cell stops dividing and renewing. Consequently, the cell is increasingly unable to perform its functions. This mechanism is one of the ways in which we age.
Telomere length is therefore regarded as a marker for the biological age of a person—in contrast to their chronological age. For two people of the same chronological age, the person with shorter telomeres has an increased risk of developing age-related diseases such as Alzheimer’s or cancer, and even a shorter life expectancy.