The National Institutes of Health has launched a program to study a rare type of cells, called “senescent” cells, that play both positive and negative roles in biological processes. The NIH Common Fund’s Cellular Senescence Network (SenNet) program will leverage recent advances in studying individual cells, or single-cell analysis, to comprehensively identify and characterize the differences in senescent cells across the body, across various states of human health, and across the lifespan. The rarity and diversity of these cells previously made them difficult to identify and study; therefore, a deeper understanding will help researchers develop therapies that encourage beneficial effects of senescent cells while suppressing their tissue-damaging effects.
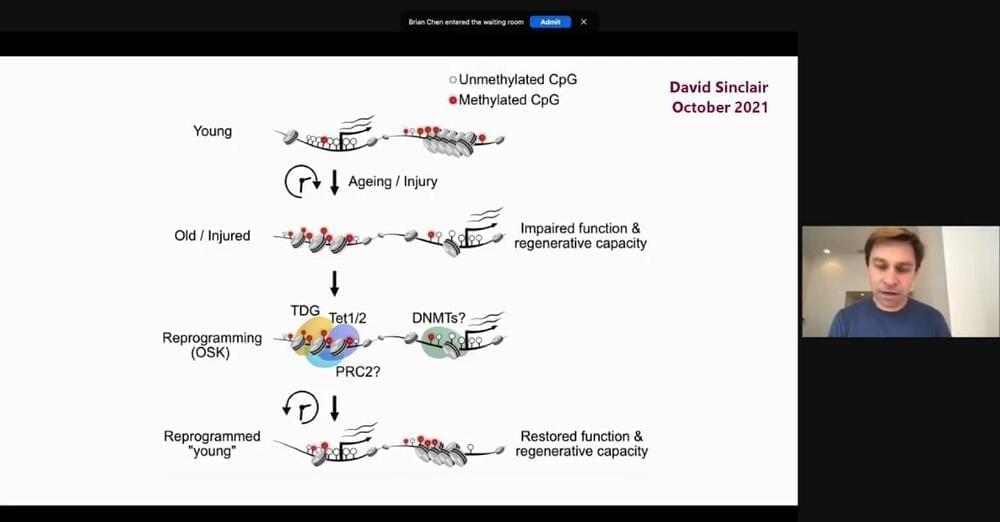
“The number of senescent cells in a person’s body increases with age, which may reflect both an increase in the generation of these cells and a decreased ability of the aging immune system to regulate or eliminate these cells. This age-related accumulation of senescent cells leads to production of inflammatory molecules and corruption of healthy cells,” said Richard J. Hodes, M.D., director of the National Institute on Aging, part of NIH. “This can affect a person’s ability to withstand stress or illness, recuperate from injuries, and maintain normal brain function. The aim of NIH’s strengthened focus on this field of science is to one day conquer these and other challenges.”
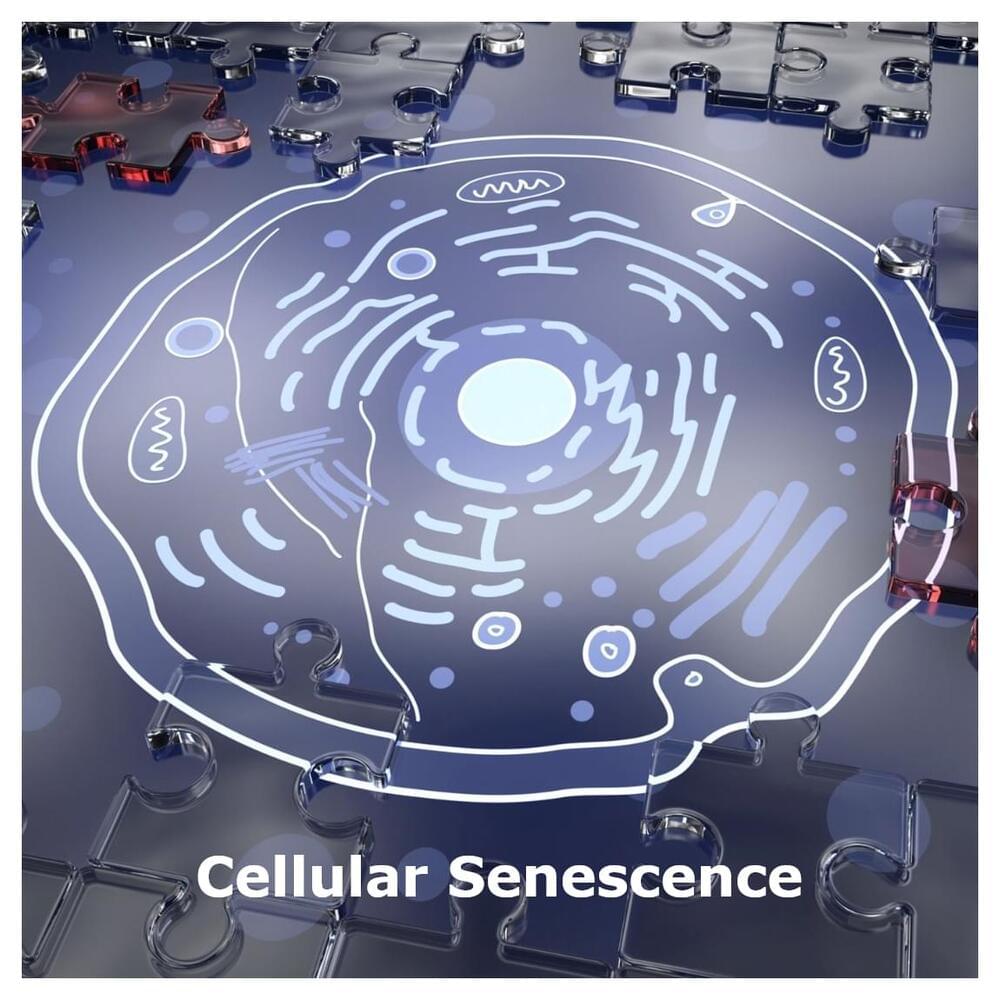
A cell dividing into two cells is a hallmark of human development. Over time, our bodies accumulate a small number of cells that no longer divide. These “senescent” cells can play important roles in health, either directly or through the release of molecules that affect neighboring cells. Senescent cells can play positive roles, such as aiding wound repair or preventing tumor growth in some cancers. However, they can also contribute to chronic diseases of aging such as cardiovascular disease and neurodegeneration. For this reason, therapeutics called “senolytics” are being developed to target senescent cells and remove them from the body.