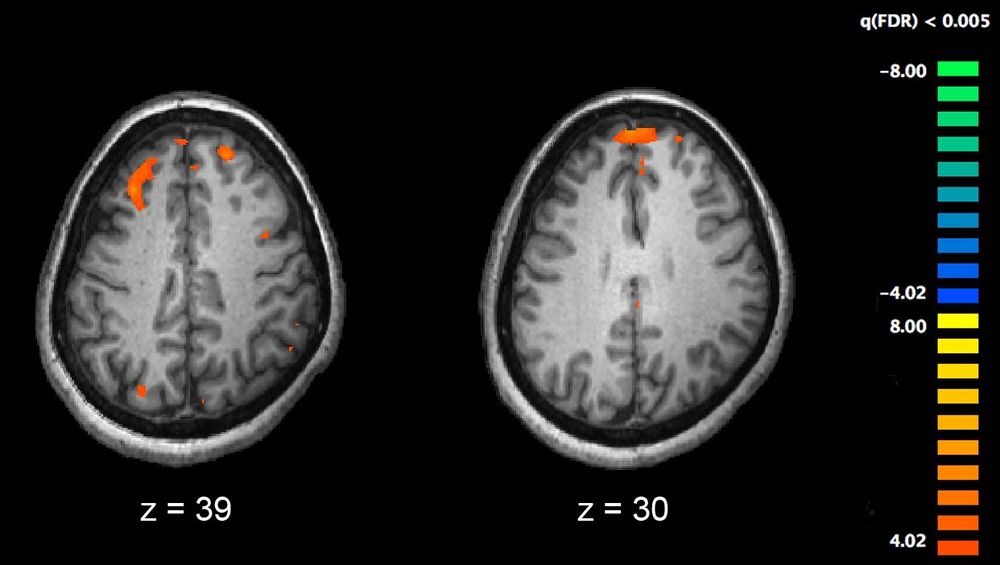
Scientists are exploring how to edit genomes and even create brand new ones that never existed before, but how close are we to harnessing synthetic life?
» Subscribe to Seeker! http://bit.ly/subscribeseeker
» Watch more How Close Are We | http://bit.ly/HCAWplaylist
» Follow Olivia on Instagram: instagram.com/OliviaPavcoG
Scientists have made major strides when it comes to understanding the base code that underlies all living things—but what if we could program living cells like software?
The principle behind synthetic biology, the emerging study of building living systems, lies in this ability to synthesize life. An ability to create animal products, individualized medical therapies, and even transplantable organs, all starting with synthetic DNA and cells in a lab.
There are two main schools of thought when it comes to synthesizing life: building artificial cells from the bottom-up or engineering microorganisms so significantly that it resynthesizes and redesigns the genome.
With genetic engineering tools becoming more and more accessible, researchers want to use these synthesized genomes to enhance human health with regards to things like detecting infections or environmental pollutants. Bacterial cells can be engineered that will detect toxic chemicals.
And these synthesized bacteria could potentially protect us from, for example, consuming toxins in contaminated water.