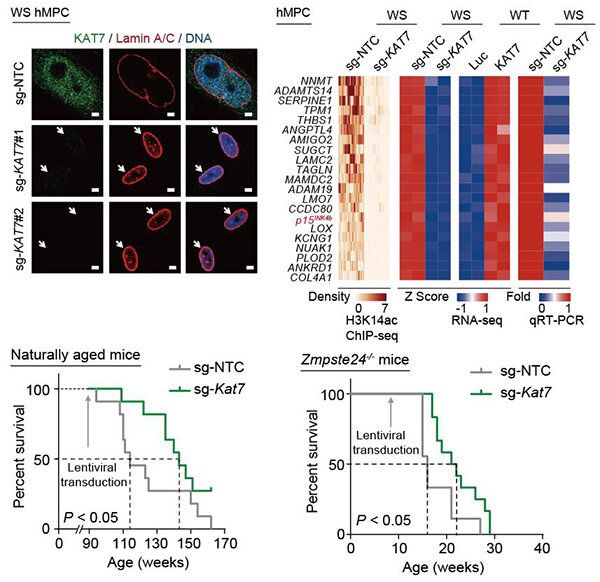
At the more advanced end of things, genetic modifications and advanced medical procedures might be available in the future that can restore muscle tissue, bone density, and organ health. If such treatments are available down the road, periodic visits to the doctor could allow Loonies to live happy and healthy lives in lower gravity.
In so many ways, a permanent human presence on the Moon could open the door to the entire Solar System. With the ability to refuel and resupply missions from a lunar site, space agencies could shave billions off the cost of deep-space missions. It would also facilitate missions to Mars, Venus, the Asteroid Belt, and beyond.