Circa 2020
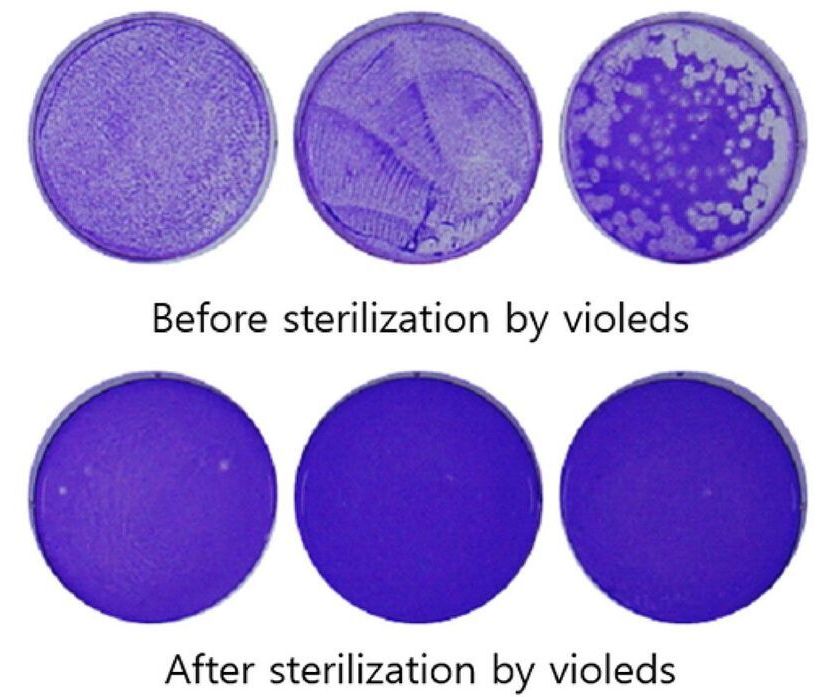
Robots and stranger machines have been using a particular band of ultraviolet light to sterilize surfaces that might be contaminated with coronavirus. Those that must decontaminate large spaces, such as hospital rooms or aircraft cabins, use large, power-hungry mercury lamps to produce ultraviolet-C light. Companies around the world are working to improve the abilities of UV-C producing LEDs, to offer a more compact and efficient alternative. Earlier this month, Seoul Viosys showed what it says is the first 99.9 percent sterilization of SARS-COV-2, the coronavirus that causes COVID-19, using ultraviolet LEDs.
UV LEDs are deadly to viruses and bacteria, because the 100–280 nanometer wavelength C-band shreds genetic material. Unfortunately, it’s also strongly absorbed by nitrogen in the air, so sources have to be powerful to have an effect at a distance. (Air is such a strong barrier, that the sun’s UV-C doesn’t reach the Earth’s surface.) Working with researchers at Korea University, in Seoul, the company showed that its Violed LED modules could eliminate 99.9 percent of the SARS-COV-2 virus using a 30-second dose from a distance of three centimeters.
Unfortunately, the company did not disclose how many of its LEDs were used to achieve that. Assuming that it and the university researchers used a single Violed CMD-FSC-CO1A integrated LED module, a 30-second dose would have delivered at most 600 millijoules of energy. This is somewhat in-line with expectations. A study of UVC’s ability to kill influenza A viruses on N95 respirator masks indicated that about 1 joule per square centimeter would do the job.