Methuselah the tree was grown from a 2000-year-old seed found at Masada. Now more have been grown and genetic analysis finds a twist in the origin story of the ‘Judean date’.


Stress management.
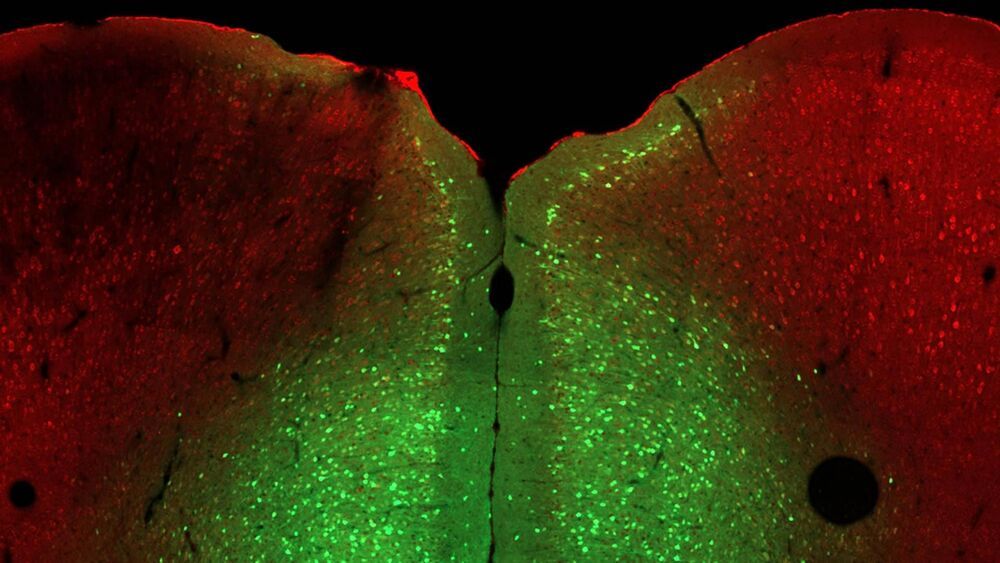
Everyone faces stress occasionally, whether in school, at work, or during a global pandemic. However, some cannot cope as well as others. In a few cases, the cause is genetic. In humans, mutations in the OPHN1 gene cause a rare X-linked disease that includes poor stress tolerance. Cold Spring Harbor Laboratory (CSHL) Professor Linda Van Aelst seeks to understand factors that cause specific individuals to respond poorly to stress. She and her lab studied the mouse gene Ophn1, an analog of the human gene, which plays a critical role in developing brain cell connections, memories, and stress tolerance. When Ophn1 was removed in a specific part of the brain, mice expressed depression-like helpless behaviors. The researchers found three ways to reverse this effect.
To test for stress, the researchers put mice into a two-room cage with a door in between. Normal mice escape from the room that gives them a light shock on their feet. But animals lacking Ophn1 sit helplessly in that room without trying to leave. Van Aelst wanted to figure out why.
Her lab developed a way to delete the Ophn1 gene in different brain regions. They found that removing Ophn1 from the prelimbic region of the medial prefrontal cortex (mPFC), an area known to influence behavioral responses and emotion, induced the helpless phenotype. Then the team figured out which brain circuit was disrupted by deleting Ophn1, creating overactivity in the brain region and ultimately the helpless phenotype.


Autistic people tend to switch between images more slowly than non-autistic people do, a previous study shows. And they spend more time seeing a combination of the two images.
Children with genetic conditions linked to autism perform atypically on a test of binocular rivalry, according to a new unpublished study.
Researchers presented the work virtually today at the 2021 International Society for Autism Research annual meetin g. (Links to abstracts may work only for registered conference attendees.)
Binocular rivalry is what happens when a person’s eyes each receive a different input. Most people gradually shift between perceiving one image and then the other as their eyes compete for dominance over the neural circuits involved.

7:01 they talk about Church’s comments of ending aging by 2030. Also this appears to be a part one.
In this video Professor Church talks about his theory of aging and touches on his ideas on the future of aging.
George Church is the Robert Winthrop Professor of Genetics at Harvard Medical School, a Professor of Health Sciences and Technology at Harvard and MIT. Professor Church helped initiate the Human Genome Project in 1984 and the Personal Genome Project in 2005. He is widely recognized for his innovative contributions to genomic science and his many pioneering contributions to chemistry and biomedicine. He has co-authored 580 paper, 143 patent publications & the book “Regenesis”.
George Church Links.
Professor Church’s Lab at Harvard.
https://arep.med.harvard.edu/
Professor Church’s Book on Amazon.
Regenesis: How Synthetic Biology Will Reinvent Nature and Ourselves.
https://amzn.to/3vTAVKo.
Twitter Link.
https://twitter.com/geochurch.
***********************************************************************************
If you would like to support our channel, we’d love a coffee…thank you! https://www.buymeacoffee.com/mhealthspan.
#TheoryOfAging #GeorgeChurch #Harvard

I still don’t get how there seems to be No organized effort anywhere to achieve the ability to 3D print a perfect genetic match of all organs by 2025 — 2030. You would think some government somewhere would want to work round the clock on this.
NIBIB-funded engineers at the University of Buffalo have fine-tuned the use of stereolithography for 3D printing of organ models that contain live cells. The new technique is capable of printing the models 10–50 times faster than the industry standard-;in minutes instead of hours-; a major step in the quest to create 3D-printed replacement organs.
Conventional 3D printing involves the meticulous addition of material to the 3D model with a small needle that produces fine detail but is extremely slow —taking six or seven hours to print a model of a human part, such as a hand, for instance. The lengthy process causes cellular stress and injury inhibiting the ability to seed the tissues with live, functioning cells.
The method developed by the SUNY Buffalo group, led by Rougang Zhao, PhD, Associate Professor of Biomedical Engineering in the Jacobs School of Medicine & Biomedical Sciences, takes a different approach that minimizes damage to live cells. The rapid, cell friendly technique is a significant step towards creating printed tissues infused with large numbers of living cells.
After doubling its sample size, the largest study of genetic data from autistic people has identified 255 genes associated with the condition, an increase of more than 40 genes since the researchers’ 2019 update; 71 of the genes rise above a stringent statistical bar the team had not previously used. The new analysis also adds data from people with developmental delay or schizophrenia and considers multiple types of mutations.
“It’s a really significant step forward in what we do,” said Kyle Satterstrom, a computational biologist in Mark Daly’s lab at the Broad Institute in Cambridge, Massachusetts. Satterstrom presented the findings virtually on Tuesday at the 2021 International Society for Autism Research annual meeting. (Links to abstracts may work only for registered conference attendees.)
The team’s previous analyses used data from the Autism Sequencing Consortium, which enrolls families through their doctors. The researchers mainly scoured the genetic data to find rare, non-inherited mutations linked to autism.

People who reach a very old age may have their genes to thank. Genetic variants that help to prevent DNA mutations and repair any that do occur have been found in supercentenarians and semi-supercentenarians – people who reach the ages of 110 and 105, respectively.
“DNA repair mechanisms are extremely efficient in these people,” says Claudio Franceschi at the University of Bologna in Italy. “It is one of the most important basic mechanisms for extending lifespan.”

The human cells grew inside all 132 of the embryos after just 24 hours. After ten days, 103 chimeric embryos remained. By day 19, however, only three chimeras were left alive – and they were then terminated.
“This knowledge will allow us to go back now and try to re-engineer these pathways that are successful for allowing appropriate development of human cells in these other animals,” Juan Carlos Izpisua Belmonte, genetics professor at the Salk Institute for Biological Sciences in La Jolla, California and co-author of the study, told NPR.
“We are very, very excited,” he added.

Genetic treatments are difficult to produce without facilities.
After Kelli Luginbuhl finished her PhD, her advisor, Duke bioengineer and PhaseBio co-founder Ashutosh Chilkoti, sat her down and asked if she wanted to launch and then run a company. Chilkoti had a once-obscure technology he and the venture capitalist Joe McMahon thought could form the basis of his second company and finally pay huge dividends. Luginbuhl knew the tech from years in his lab and was already looking for biotech jobs. It all added up.
Three years, some strategizing, and 10 or so pitch meetings later, the trio is launching Isolere Bio, with $7 million in seed funding led by Northpond Ventures and technology they believe can allow gene therapy companies to vastly increase the number of doses they can produce. It’s one potential solution to a slow-boiling crisis that has become increasingly acute, as new companies struggle to get the materials they need for trials and some common diseases remain theoretically unfixable by gene therapy, because companies would never be able to make enough doses for that many patients.
The problem is partially that the facilities don’t yet exist to produce this much of gene therapy. Experts, however, also point to antiquated manufacturing processes.