The artist’s remains are reportedly buried in France’s Chateau d’Amboise. Now, scientists may finally be about to identify them.


😀 This could to more complex organisms being resurrected.
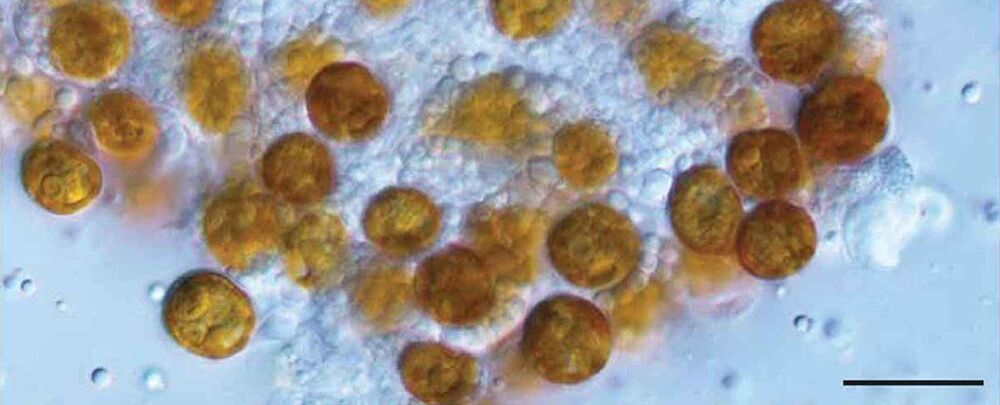
Deep in the tissues of sea anemones, corals, and jellyfish are strange yellow cells which are genetically distinct from the marine animals.
More than a century after these cells were first assigned a now forgotten genus, a new paper has resurrected the name and described six new species from around the world.
“Because our team comprises scientists from seven countries, we were able to collect all of these samples, and some during the global pandemic,” said lead author of the study, biologist Todd LaJeunesse from Penn State University.
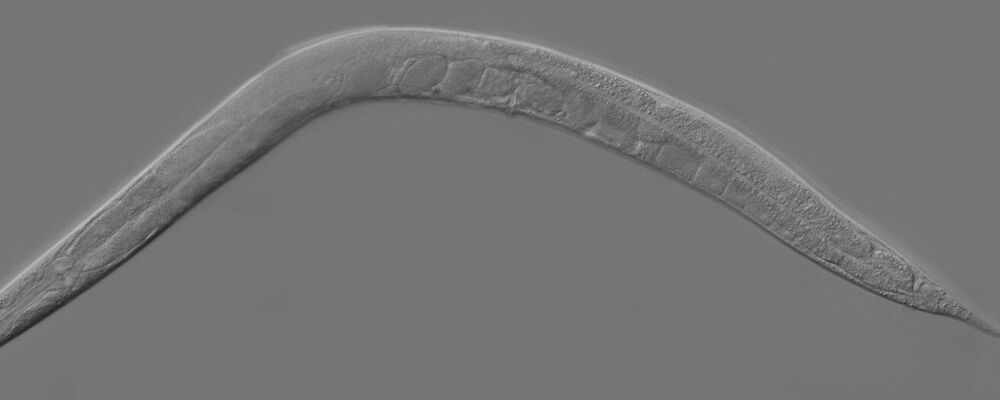
Most biological traits are inherited via genes, but there are exceptions to this rule. Two teams from the University of Geneva (UNIGE) have been investigating the location of centromeres—specific sites on chromosomes that are essential for cell division. They found that in the small worm Caenorhabiditis elegans, the transmission of the correct location of these sites to the offspring is not mediated by genes, but by an epigenetic memory mechanism. These results have been published in the journal PLOS Biology.
Living organisms, from humans to microscopic worms, inherit physical and sometimes behavioral traits from their parents. The transmission of biological traits is usually mediated by DNA which is replicated at each cell division and contains the genes. However, some characteristics can be transmitted from one generation to the next independently of genes: these are epigenetic phenomena.
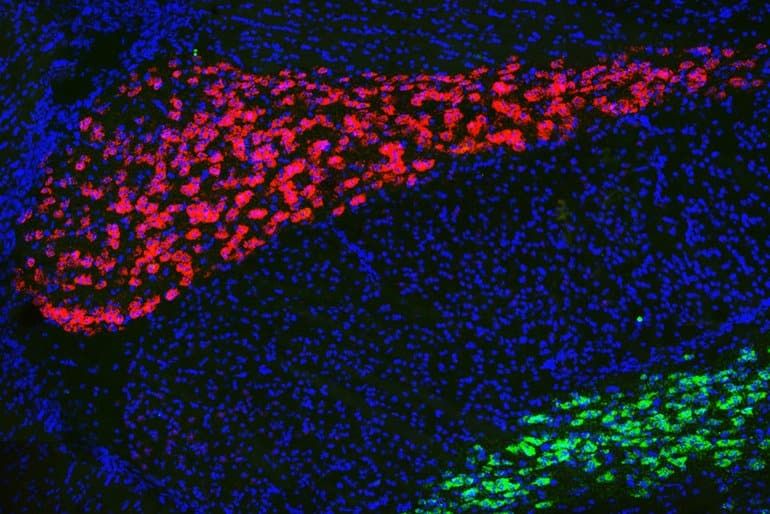
The researchers also showed that they could restore normal cognitive function in mice with these genetic mutations by artificially turning down hyperactivity in neurons of the AD thalamus. The approach they used, chemogenetics, is not yet approved for use in humans. However, it may be possible to target this circuit in other ways, the researchers say.
Summary: Certain genes that are mutated or missing in those with schizophrenia and autism cause similar dysfunction in neural networks within the thalamus.
Source: MIT
Many neurodevelopmental disorders share similar symptoms, such as learning disabilities or attention deficits. A new study from MIT has uncovered a common neural mechanism for a type of cognitive impairment seen in some people with autism and schizophrenia, even though the genetic variations that produce the impairments are different for each condition.
In a study of mice, the researchers found that certain genes that are mutated or missing in some people with those disorders cause similar dysfunctions in a neural circuit in the thalamus. If scientists could develop drugs that target this circuit, they could be used to treat people who have different disorders with common behavioral symptoms, the researchers say.

Researchers have finally sequenced the complete human genome, filling the gaps in the Human Genome Project’s (HGP) historic first draft.
“Having been part of the original Human Genome Project in 2001, and especially focused on the difficult regions, it’s really satisfying for me to see this done even though it took 20 years,” researcher Evan Eichler, a genome scientist from the University of Washington in Seattle, told New Scientist.
The human genome: A genome is like a genetic instruction manual — it contains all the information an organism needs to grow and function. The human genome is written in DNA, and while your exact genome is unique to you, about 99.9% of it is identical across all people.
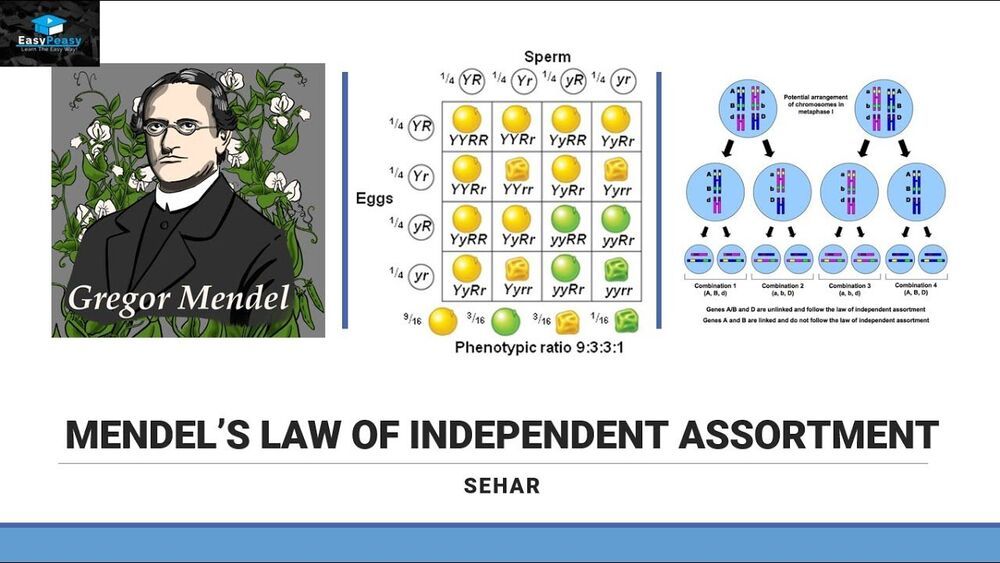
#mendelslawofindependentassortment #Genetics #genes #molecularbiology #biology #biotech #recombinants #Genetic
This video explains the mendel’s law of independent assortment.
Thank You For Watching.
Please Like And Subscribe to Our Channel: https://www.youtube.com/EasyPeasyLearning.
Like Our Facebook Page: https://www.facebook.com/learningeasypeasy/
Join Our Facebook Group: https://www.facebook.com/groups/460057834950033
Support Our Channel: https://www.patreon.com/supereasypeasy
Not sure how interesting this will be to people who know a lot on aging/longevity research.
A team of researchers at Brigham and Women’s Hospital and Harvard Medical School have found evidence of mouse and human germline cells resetting their biological age. In their paper published in the journal Science Advances, the group describes their study of the aging process in germline cells and what they found by doing so.
As animals grow older, all of the cells in their body replicate themselves repeatedly. As the process continues, errors in replicating and other external factors (such as exposure to pollutants) lead to gradual decay in cell quality, which is all part of the natural aging process. In this new effort, the researchers have found evidence showing that germline cells have a mechanism for resetting this process, allowing offspring to reset their aging clocks.
Germline cells pass on genetic material from parent to offspring during the reproductive process. For many years, scientists have wondered why these cells do not inherit the age of their parents. And for many years, they assumed that the cells were ageless, but recent work has shown that they do, in fact, age. So that raised the question of how offspring are able to begin their lives with fresh cells.
Researchers from Brigham and Women’s Hospital have engineered yeast used in baking, wine-making and brewing to treat inflammatory bowel disease (IBD). The bacteria has been modified to secrete an anti-inflammatory molecule in response to signs of gut inflammation and has proven effective in preclinical tests.
Our gut microbiome is increasingly implicated in everything from cancer to neurodegenerative disease but it is still unclear exactly how we can translate these novel findings into clinical treatments. Fecal transplants are probably the most primitive microbiome-modifying treatment we have developed, while probiotics simply rely on upping specific levels of naturally occurring bacteria.
Perhaps the most futurist microbiome therapy under investigation is the idea of genetically engineered probiotics. Here researchers modify bacteria to either eat up molecules we don’t want in our body or secrete molecules we know have positive therapeutic effects.
I was at HudsonAlpha’s spinoff clinic for rare diseases, the Smith Family Clinic for Genomic Medicine. Most people don’t know this, but the second largest biomedical research campus in the USA and the fourth in the entire world is in Alabama. Long-read genome sequencing is essential for aging research because it is able to detect methylation and acetylation very conveniently, as well as major structural changes to the genome that are associated with both rare disease AND aging. This is an explanation of how long-read sequencing is able to fill in sequence gaps caused by Illumina short-read technology.
In 2020, Chromosome X and 8 were finished end-to-end with long-read sequencing, for the first time. And now in 2021, a complete gapless human genome is on the horizon. The Human Genome Project may finally, truly become complete.
February 3, 2021 (Huntsville, Ala.) – Researchers at the HudsonAlpha Institute for Biotechnology used a new, cutting-edge genomic sequencing technology to help physicians make diagnoses for two pediatric patients who had been on long diagnostic journeys.
Limitations of traditional sequencing in neurodevelopmental disease diagnosis
Neurodevelopmental diseases, many of which are genetic in nature, affect one to three percent of children and cause a range of physical and intellectual disabilities. Identifying the genetic variants, or changes in DNA, that lead to these diseases can provide a precise diagnosis, guide treatment approaches, and give families the answer to their years-long medical mystery.

Big fan of long-read sequencing. It helped diagnose my rare disease when conventional sequencing failed.
What’s the Difference between Short-Read Sequencing and Long-Read Sequencing? Like their names suggest, short-read sequencing looks at DNA in short snippets (100−350 base pairs) while long-read sequencing measures long fragments of DNA (tens of thousands of base pairs). Why does that matter? Well, when trying to characterize a human genome that has two copies (one maternal and one paternal), each 3.2 billion base pairs in length — having longer snippets of DNA means you: Need fewer snippets to make up the length of the whole genome and have no gaps where the sequence is unknown Can more easily map how one region of the genome is connected to another region Have the ability to phase or determine which copy of a gene, maternal or paternal, a mutation occurs in.
PacBio long-read sequencing provides the most comprehensive view of genomes, transcriptomes, and epigenomes.