A technique based on genetic bar codes can easily map the connections of individual brain cells in unprecedented numbers. Unexpected complexity in the visual system is only the first secret it has revealed.

A 10-year-long study called the PanCancer Atlas is releasing a trove of genetic data in an effort to help doctors treat a wide variety of cancers more precisely.
The history: Over the past decade, 150 researchers from the US and around the world painstakingly analyzed DNA, RNA, and proteins from tumor samples of more than 11,000 patients with 33 different types of cancer.
The findings: From that data, scientists have identified about 300 genes that drive tumor growth. They also found that just over half the tumors samples analyzed carry genetic mutations that could be targeted by therapies that are already on the market. These findings and others appear in 29 different papers today in the journal Cell.
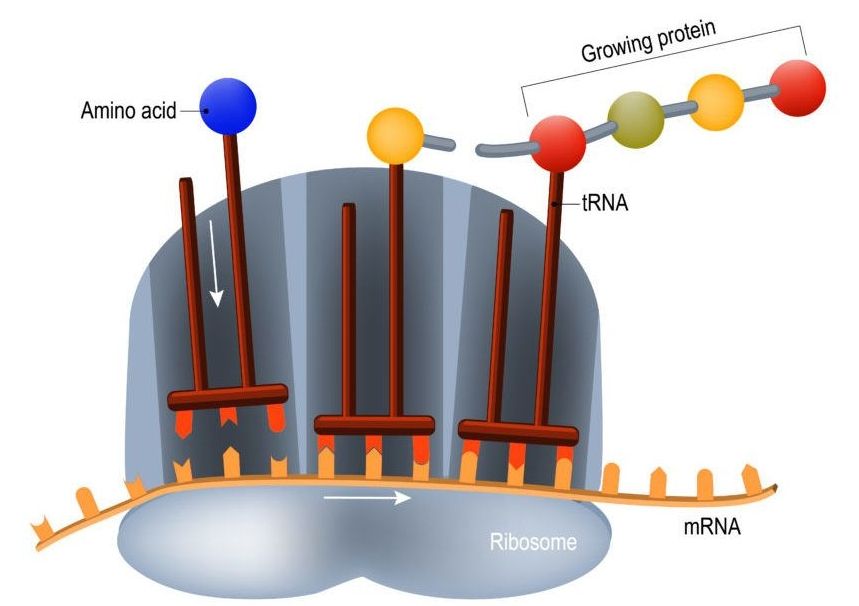
Protein synthesis is a critical part of how our cells operate and keep us alive and when it goes wrong it drives the aging process. We take a look at how it works and what happens when things break down.
Suppose that your full-time job is to proofread machine-translated texts. The translation algorithm commits mistakes at a constant rate all day long; from this point of view, the quality of the translation stays the same. However, as a poor human proofreader, your ability to focus on this task will likely decline throughout the day; therefore, the number of missed errors, and therefore the number of translations that go out with mistakes, will likely go up with time, even though the machine doesn’t make any more errors at dusk than it did at dawn.
To an extent, this is pretty much what is going on with protein synthesis in your body.
Protein synthesis in a nutshell
The so-called coding regions of your DNA consist of genes that encode the necessary information to assemble the proteins that your cells use. As your DNA is, for all intents and purposes, the blueprint to build you, it is pretty important information, and as such, you want to keep it safe. That’s why DNA is contained in the double-layered membrane of the cell nucleus, where it is relatively safe from oxidative stress and other factors that might damage it. The protein-assembling machinery of the cell, ribosomes, are located outside the cell nucleus, and when a cell needs to build new proteins, what’s sent out to the assembly lines is not the blueprint itself, but rather a disposable mRNA (messenger RNA) copy of it that is read by the ribosomes, which will then build the corresponding protein. The process of making an mRNA copy of DNA is called “translation”, and as the initial analogy suggests, it is not error-free.

The initiative, which launched on March 20, will start by providing 100,000 of its 1.3 million residents with information on their genetic risk for certain diseases. Genetic information from the project will first be delivered to a family doctor, so that patients will receive counseling about what their results actually mean and how they can better adapt their lifestyle to avoid illness.
The nation of Estonia is establishing a program that provides both free genetic testing and health advice to all citizens based on their results.

Advocates of transhumanism face a similar choice today. One option is to take advantage of the advances in nanotechnologies, genetic engineering and other medical sciences to enhance the biological and mental functioning of human beings (never to go back). The other is to legislate to prevent these artificial changes from becoming an entrenched part of humanity, with all the implied coercive bio-medicine that would entail for the species.
We can either take advantage of advances in technology to enhance human beings (never to go back), or we can legislate to prevent this from happening.



Today we bring you an interview with Professor Steve Horvath pioneer of the epigenetic clocks of aging.
Steve Horvath is a Professor of Human Genetics and Biostatistics at UCLA. His research sits at the intersection of biostatistics, bioinformatics, computational biology, cancer research, genetics, epidemiology, epigenomics, machine learning, and systems biology.

The discovery of a genetic switch that triggers stem cells to turn into heart cells is a major step in finding treatment for damaged hearts.
Researchers from A*STAR and their colleagues in India have been investigating the molecular and genetic processes by which human embryonic stem cells differentiate into the body’s many types of cells—in particular, cardiomyocytes, or heart muscle cells.
“The effort is underway globally to find ways to differentiate these stem cells into beating functional heart muscle cells so that they can be used for cell-based therapies to treat structural abnormalities,” says Prabha Sampath, from the A*STAR Institute of Medical Biology.