They used a gene drive to create a genetic time bomb.


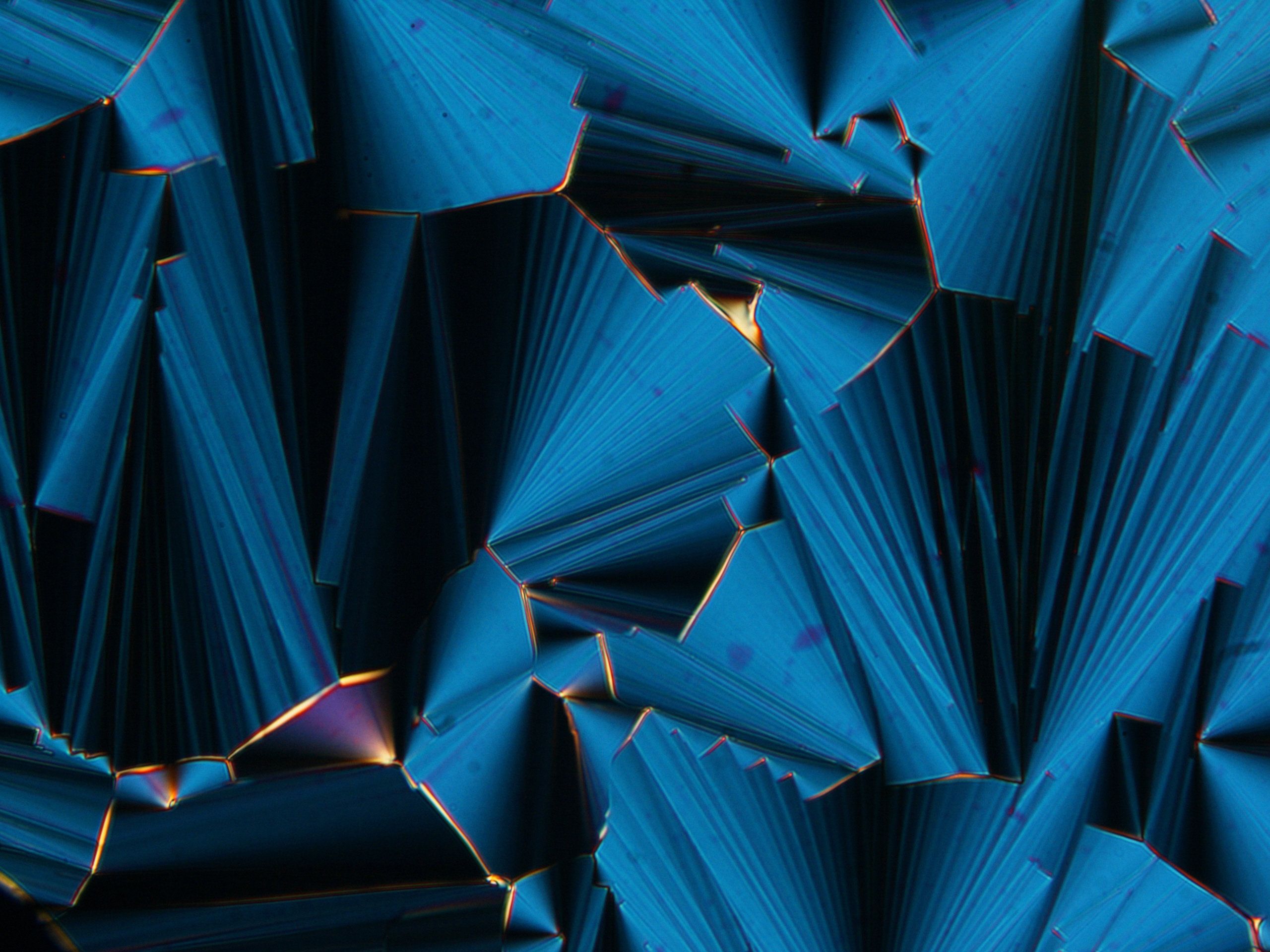
The display screens of modern televisions, cell phones and computer monitors rely on liquid crystals—materials that flow like liquids but have molecules oriented in crystal-like structures. However, liquid crystals may have played a far more ancient role: helping to assemble Earth’s first biomolecules. Researchers reporting in ACS Nano have found that short RNA molecules can form liquid crystals that encourage growth into longer chains.
Scientists have speculated that life on Earth originated in an “RNA world,” where RNA fulfilled the dual role of carrying genetic information and conducting metabolism before the dawn of DNA or proteins. Indeed, researchers have discovered catalytic RNA strands, or “ribozymes,” in modern genomes. Known ribozymes are about 16–150 nucleotides in length, so how did these sequences assemble in a primordial world without existing ribozymes or proteins? Tommaso Bellini and colleagues wondered if liquid crystals could help guide short RNA precursors to form longer strands.
To find out, the researchers explored different scenarios under which short RNAs could self-assemble. They found that at high concentrations, short RNA sequences (either 6 or 12 nucleotides long) spontaneously ordered into liquid crystal phases. Liquid crystals formed even more readily when the researchers added magnesium ions, which stabilized the crystals, or polyethylene glycol, which sequestered RNA into highly concentrated microdomains. Once the RNAs were held together in liquid crystals, a chemical activator could efficiently join their ends into much longer strands. This arrangement also helped avoid the formation of circular RNAs that could not be lengthened further. The researchers point out that polyethylene glycol and the chemical activator would not be found under primordial conditions, but they say that other molecular species could have played similar, if less efficient, roles.

The field of epigenetics sits precariously on the precipice of the classic nature versus nurture debate. Instead of a simple environment versus genetics dichotomy, epigenetic examines how specific genes are either switched on or off through external forces encountered in a person’s lifetime. Striking new research from scientists at the University of British Columbia and Harvard University is suggesting that adults who were victims of abuse as children may carry an imprint of that trauma in regions of their DNA.

Today, we would like to share with you a talk by Dr. Michael West from AgeX Therapeutics, a company developing therapies to combat age-related diseases by encouraging the body to regenerate cells and tissues.
On July 12th, we hosted our first conference, Ending Age-Related Diseases: Investment Prospects & Advances in Research, at the Frederick P. Rose Auditorium, which is part of the Cooper Union campus in New York City. The packed event saw a range of people from research, investment, and the wider community coming together for a day of science and biotech business presentations and panels.
In his talk, “Hayflick Rewound: Somatic Restriction, Epigenetics, and the Reversibility of Human Aging”, Dr. Michael West, CEO of AgeX Therapeutics, discussed the breakthroughs in our understanding of biological regeneration and in induced tissue regeneration.

Be gone flat earthism.
The nature-versus-nurture argument of intelligence just got a lot more complicated with the discovery that the environment can modify the expression of a key gene in the brain, affecting intelligence far more than we previously thought.
Such a finding may not come as a surprise if you remember that numerous genes influence our IQ and stressful experiences can lock and unlock genes in our brains. Yet having hard evidence of the link will no doubt stir debate on just what it means to be “smart”.
Researchers from the Charité — Universitätsmedizin Berlin analysed the characteristics of a number of genes among a group of healthy adolescents, and compared the results with intelligence scores and various neurological traits.

👀
The GTPases constitute a very large protein family, whose members are involved in the control of cell growth, transport of molecules, synthesis of other proteins, etc. Despite the many functions of the GTPases, they follow a common cyclic pattern (Figure 1). The activity of the GTPases is regulated by factors that control their ability to bind and hydrolyse guanosine triphosphate (GTP) to guanosine diphosphate (GDP). So far, it has been the general assumption that a GTPase is active or “on” when it is bound to GTP and inactive or “off” in complex with GDP. The GTPases are therefore sometimes referred to as molecular “switches.”
The bacterial translational elongation factor EF-Tu is a GTPase, which plays a crucial role during the synthesis of proteins in bacteria, as the factor transports the amino acids that build up a cell’s proteins to the cellular protein synthesis factory, the ribosome. Previous structural studies using X-ray crystallography have shown that EF-Tu occurs in two markedly different three-dimensional shapes depending on whether the factor is “on” (i.e. bound to GTP) or “off” (i.e. bound to GDP) (Figure 2). The binding of GTP/GDP have therefore always been thought to be decisive for the factor’s structural conformation.
However, a research collaboration between researchers from the Department of Molecular Biology and Genetics at Aarhus University and two American universities reveals that EF-Tu’s structure and function, and probably also those of other GTPases, are far more complex than previously assumed. In Søren Thirup’s group, X-ray crystallographic analysis of E. coli EF-Tu has shown that EF-Tu bound to a variant of GTP, GDPNP, can also occur in the “off” state, which is characterised by a more open structure. In collaboration with American researchers, Charlotte Knudsen’s Ph.D. student, Darius Kavaliauskas, conducted further studies using a special form of fluorescence microscopy that makes it possible to observe the spatial structure of individual EF-Tu molecules in solution.


A recent open-access mouse study published by Xi’an Institute of Tissue Engineering and Regenerative Medicine scientists in the journal Bone Research describes how the ALPL gene affects bone aging and suggests that metformin might constitute a viable therapeutic option to prevent it [1].
Study abstract
Mutations in the liver/bone/kidney alkaline phosphatase (Alpl) gene cause hypophosphatasia (HPP) and early-onset bone dysplasia, suggesting that this gene is a key factor in human bone development. However, how and where Alpl acts in bone ageing is largely unknown. Here, we determined that ablation of Alpl induces prototypical premature bone ageing characteristics, including bone mass loss and marrow fat gain coupled with elevated expression of p16INK4A (p16) and p53 due to senescence and impaired differentiation in mesenchymal stem cells (MSCs). Mechanistically, Alpl deficiency in MSCs enhances ATP release and reduces ATP hydrolysis. Then, the excessive extracellular ATP is, in turn, internalized by MSCs and causes an elevation in the intracellular ATP level, which consequently inactivates the AMPKα pathway and contributes to the cell fate switch of MSCs.

That conclusion is based on a study that reviewed genetic testing results from 1.45 million individuals and found that nearly 25 percent of “variants of uncertain significance” were subsequently reclassified — sometimes as less likely to be associated with cancer, sometimes as more likely.
The study appears in the Journal of the American Medical Association (JAMA).
When variations from the norm are discovered in a gene, the variants are classified as “benign,” “likely benign,” “variant of uncertain significance,” “likely pathogenic,” or “pathogenic.”