Category: genetics

This woman’s genetic mutation shields her from pain and anxiety
Arthritis is usually painful. So is the surgery to fix it, at least in the immediate aftermath. So when a 66-year old woman at Raigmore Hospital in Inverness, Scotland, told doctors that her severely arthritic hand felt fine both before and after her operation, they were suspicious. The joint of her thumb was so severely deteriorated that she could hardly use it—how could that not hurt?
So they sent her to see teams specializing in pain genetics at University College London and the University of Oxford. Those researchers took DNA samples from both her and some of her family members and uncovered her secret: a tiny mutation in a newly-discovered gene. They recently published their results in the British Journal of Anaesthesia.
This minuscule deletion is inside something called a pseudogene, which is a partial copy of a fully functioning gene inserted elsewhere in the genome. Pseudogenes don’t always have a function—sometimes they’re just junk DNA—but some of them have residual functionality leftover from the original gene’s purpose.

The biggest revolution in gene editing: Crispr-Cas9 explained – video
Prof Jennifer Doudna, one the pioneers of Crispr-Cas9 gene editing, explains how this revolutionary discovery enables precise changes to our DNA, which can be used to correct mutations that cause genetic diseases and eradicate them from a germ line. Doudna raises the key issues of debate around gene editing and suggests what will have the most immediate impact.

Gene-Editing Record Smashed With Over 13,000 Changes Made to a Single Human Cell
Using a modified version of CRISPR, a team of geneticists has successfully triggered 13,200 genetic changes to a single human cell. That’s a new record, by a long shot. This sweeping new editing process could eventually be used to strip DNA of useless or dangerous genetic information—or create entirely new kinds of life.
New research uploaded to the preprint bioRxiv server describes the achievement, in which a Harvard University team led by George Church edited the living crap out of a single human cell to the tune of 13,200 total modifications. Incredibly, the cell survived. The previous record for bulk edits made to a single cell was set in 2017, when Church and his colleagues knocked out 62 copies of a retrovirus found in pig genomes. The new achievement is thus “three orders of magnitude greater” than the previous standard, the authors wrote in their paper.
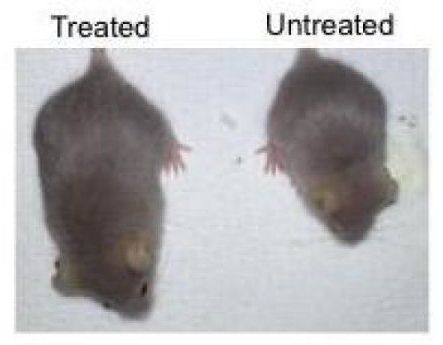
CRISPR/Cas9 therapy can suppress aging, enhance health and extend life span in mice
The findings, published on February 18, 2019 in the journal Nature Medicine, highlight a novel CRISPR/Cas9 genome-editing therapy that can suppress the accelerated aging observed in mice with Hutchinson-Gilford progeria syndrome, a rare genetic disorder that also afflicts humans. This treatment provides important insight into the molecular pathways involved in accelerated aging, as well as how to reduce toxic proteins via gene therapy.
“Aging is a complex process in which cells start to lose their functionality, so it is critical for us to find effective ways to study the molecular drivers of aging,” says Juan Carlos Izpisua Belmonte, a professor in Salk’s Gene Expression Laboratory and senior author of the paper. “Progeria is an ideal aging model because it allows us to devise an intervention, refine it and test it again quickly.”
With an early onset and fast progression, progeria is one of the most severe forms of a group of degenerative disorders caused by a mutation in the LMNA gene. Both mice and humans with progeria show many signs of aging, including DNA damage, cardiac dysfunction and dramatically shortened life span. The LMNA gene normally produces two similar proteins inside a cell: lamin A and lamin C. Progeria shifts the production of lamin A to progerin. Progerin is a shortened, toxic form of lamin A that accumulates with age and is exacerbated in those with progeria.
NMN, NAD+ and the Plasma Membrane
Earlier this year, we hosted the Ending Age-Related Diseases 2018 conference at the Cooper Union, New York City. This conference was designed to bring together the best in the aging research and biotech investment worlds and saw a range of industry experts sharing their insights.
Joe Betts Lacroix of Y Combinator and Vium discusses the different ways in which entrepreneurs can focus on overcoming the diseases of aging, namely direct, indirect, and money-first approaches, and the strengths and weakness of each.
Joe was the primary technical founder of hardware/software startup OQO, which entered the Guinness Book of World Records for building the smallest fully featured PC. His experience spans from biotech research to electronics design. Very experienced in invention, prosecution and monetization of intellectual property, he has over 80 patents granted and pending in fields ranging from biophysics and safety systems to antennas, thermal systems, user interfaces, and analog electronics. He has written numerous peer-reviewed publications in fields such as biophysics, genetics, electronics, and robotics. Joe holds a Harvard A.B., an MIT S.M. and a Caltech research fellowship.

Local extinction of Southern California mountain lions possible within 50 years
Two isolated mountain lion populations in southern California’s Santa Ana and Santa Monica Mountains are at risk of local extinction, perhaps as soon as within 50 years, according to a study published in the journal Ecological Applications.
The study showed the extinction risk is due to low genetic diversity and mortality that affects the stability of the population. Mountain lion mortality is often caused by humans, but can also result from changes in the environment, such as wildfire and fluctuations in prey density.
The two mountain lion populations in the human-dominated landscape of southern California are isolated by freeways and development. For the study, lead author John Benson of the University of Nebraska and co-authors at UCLA, the University of California, Davis, the National Park Service, the University of Washington, Northern Arizona University, and the University of Wyoming used population viability modeling to predict the possibilities of extinction from genetic and demographic risk factors.