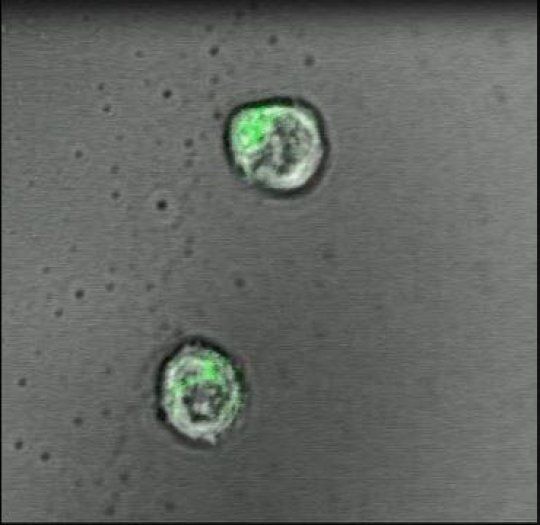
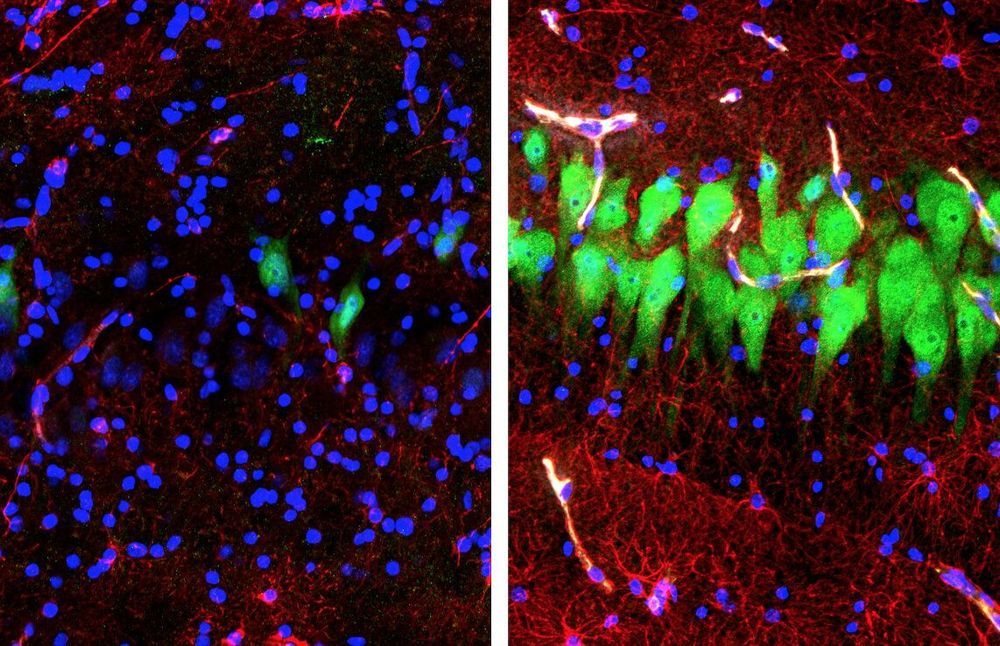
A special focus on rogue proteins may hold future promise in stopping the progression of nerve cell destruction in people who have amyotrophic lateral sclerosis (ALS) or frontotemporal dementia.
ALS, a rare but devastating disorder that’s also known as Lou Gehrig’s disease, attacks the body’s nerve cells, resulting in progressive muscle weakness as the neurons degenerate over time. There is no cure. People with ALS eventually lose their strength and the ability to move their arms, legs and body.
About a third of those with ALS also develop frontotemporal dementia (FTD), a destruction of neurons in the brain that causes profound personality changes and disability. The two diseases are similar in both pathology and genetics. FTD tends to affect people earlier than Alzheimer’s disease, the most common type of dementia.