BAE Systems is working on a form of atmospheric shield to protect against directed energy weapons.


BAE Systems is working on a form of atmospheric shield to protect against directed energy weapons.

Australian Research opens up new possibilities for hydrogen fuelled future.
Scientists show how using only water, iron, nickel and electricity can create hydrogen energy much more cheaply than before.
Hydrogen-powered cars may soon become more than just a novelty after a UNSW-led team of scientists demonstrated a much cheaper and sustainable way to create the hydrogen required to power them.
In research published in Nature Communications recently, scientists from UNSW Sydney, Griffith University and Swinburne University of Technology showed that capturing hydrogen by splitting it from oxygen in water can be achieved by using low-cost metals like iron and nickel as catalysts, which speed up this chemical reaction while requiring less energy.
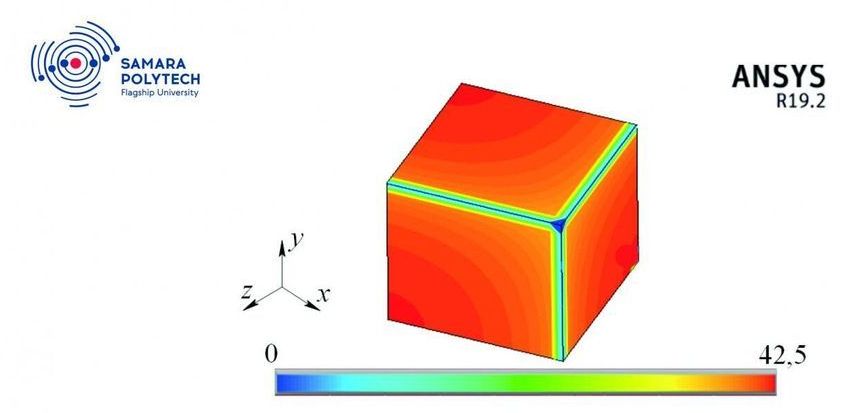
A team of scientists from the Research Center “Fundamental Problems of Thermophysics and Mechanics,” of Samara Polytech is engaged in the construction of new mathematical models and the search for methods for their study in relation to a wide range of local nonequilibrium transport processes in various physical systems. An innovative approach developed not so long ago is based on a modern version of third-generation thermodynamics. The project of these scientists, “Development, theoretical research and experimental verification of mathematical models of oscillatory processes, heat and mass transfer and thermomechanics with two- and multiphase delays” was among the winners of the RFBR contest. Recent research results are published in the journal Physica A: Statistical Mechanics and its Applications.
An interest in studying local nonequilibrium processes that take into account the specifics of transport processes at the molecular level (the mean free path of a molecule, the momentum transfer rate, relaxation time, etc.) is dictated by the need to conduct various physical processes under extreme conditions—for example, femtosecond concentrated exposure to energy flows on matter, ultra-low and ultra-high temperatures and pressures, shock waves, etc. Such physical processes are widely used to create new technologies for producing nanomaterials and coatings with unique physicochemical properties that cannot be obtained by traditional methods (binary and multicomponent metal alloys, ceramics, polymeric materials, metal and semiconductor glasses, nanofilms, graphene, composite nanomaterials, etc.).
“Classical thermodynamics is not suitable for describing processes that occur under local nonequilibrium conditions, since it is based on the principle of local equilibrium. Our project is important both for fundamental science and for practical applications,” explains the project manager, Professor Igor Kudinov. “To accomplish the tasks, we plan to create a new, unparalleled software package designed for 3D modeling of high-speed local nonequilibrium processes of heat, mass and momentum transfer. Thus, our method opens up wide possibilities for studying processes that are practically significant from the point of view of modern nanotechnology.”


And a means coming for 24 hour power supply.
First announced in 2012 and with a scheduled completion date of 2030, the 5,000-megawatt solar park will take three times as long to finish as the Burj Khalifa. Phases one and two, which are already complete, comprised 2.3 million photovoltaic panels with a capacity of 213 megawatts. Phase three, deep in construction, adds over 3 million photovoltaics and another 800 megawatts, and will be completed in 2020, say DEWA.

This 45-ton dump truck ascends a 13-percent grade and can take on 65 tons while doing so.
As the heavy transport descends with more than double the weight, the trucks regenerative braking system recaptures all the energy it will need to refill the charge that it will need to use on the way back up again. Regenerative braking allows the eDumper to produce more energy downhill than it consumes uphill.”
The eDumper is the world’s largest electric vehicle but it generates more energy than it uses so it never needs charging.

Today, IBM Research is building on a long history of materials science innovation to unveil a new battery discovery. This new research could help eliminate the need for heavy metals in battery production and transform the long-term sustainability of many elements of our energy infrastructure.
As battery-powered alternatives for everything from vehicles to smart energy grids are explored, there remain significant concerns around the sustainability of available battery technologies.
Many battery materials, including heavy metals such as nickel and cobalt, pose tremendous environmental and humanitarian risks. Cobalt in particular, which is largely available in central Africa, has come under fire for careless and exploitative extraction practices.1

