The money will go towards fuel cells powerful enough to fly a passenger plane.




Hydropower has been around for more than a century, and is currently the nation’s largest source of clean, domestic, renewable electricity. What could its role look like in the year 2050?
Providing about 7 percent of the nation’s electricity, hydropower supports more than 143, 000 jobs in engineering, manufacturing, construction and utility operations and maintenance — all while improving the environment and strengthening our economy. Additionally, pumped-storage hydropower represents 97 percent of all energy storage in the United States, offering the flexibility and reliability the electricity grid needs to deliver affordable clean energy to American homes and businesses.
So what does the future of hydropower look like? To answer that question, over the past two years the Energy Department has collaborated with more than 300 experts from more than 150 hydropower industry companies, environmental organizations, state and federal governmental agencies, academic institutions, electric power system operators, research institutions and other stakeholders to explore how it could evolve in the coming decades.

BOEM is responsible for offshore renewable energy development in Federal waters. The program began in 2009, when the Department of the Interior (DOI) announced the final regulations for the Outer Continental Shelf (OCS) Renewable Energy Program, which was authorized by the Energy Policy Act of 2005 (EPAct). These regulations provide a framework for all of the activities needed to support production and transmission of energy from sources other than oil and natural gas. BOEM anticipates future development on the OCS from these general sources:
Offshore Wind Energy
Offshore wind is an abundant, domestic energy resource that is located close to major coastal load centers. It provides an efficient alternative to long-distance transmission or development of electricity generation in these land-constrained regions.
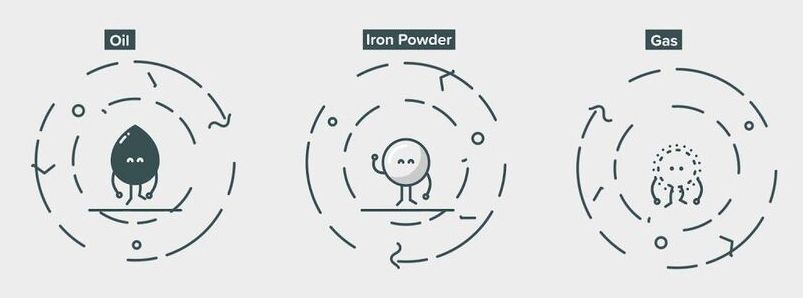
Did you know iron could burn? The combustion of iron powder seen here is taking place on an entirely smokeless, carbon free basis.
Tested in microgravity aboard ESA sounding rockets by a team from McGill University in Canada and Eindhoven University of Technology in the Netherlands, this technique has now been harnessed by Swinkels Family Brewers in the Netherlands, helping to free their brewing process from reliance on fossil fuels.
“The best way to reduce carbon emissions into the atmosphere is not to emit it at all,” explains ESA engineer Antonio Verga, who worked on flying the team’s experiments aboard TEXUS sounding rockets.

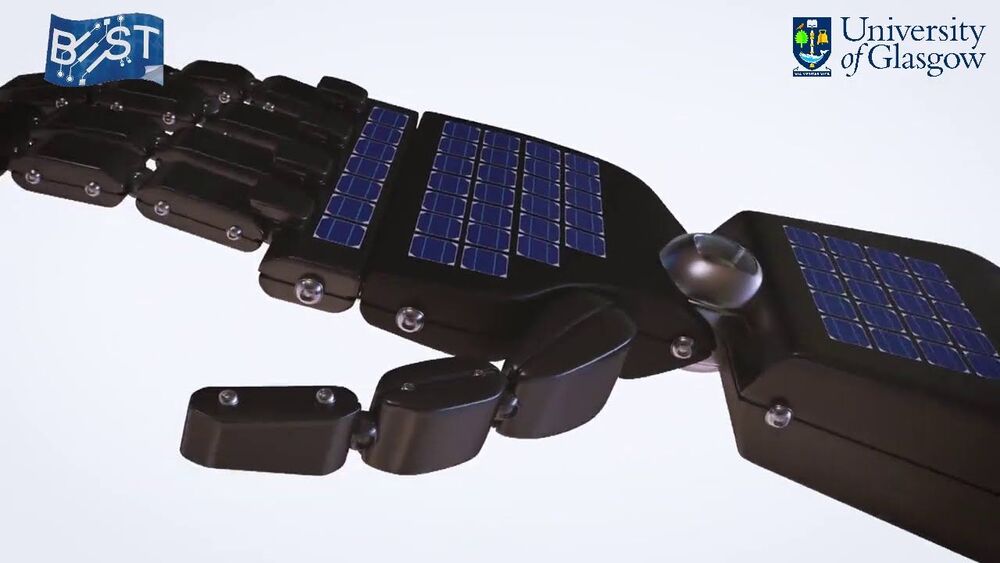
Scientists demonstrate a innovative e-skin with touch and proximity-sensing capabilities without using dedicated touch sensors.

“What our technology does is it improves range and lowers vehicle cost,” Campbell said. “It’s as simple as that.”
As the name of his company suggests, Campbell thinks the key is a more-solid electric car battery. The lithium-ion batteries powering almost all of today’s electric vehicles rely on a liquid electrolyte, which ferries charged ions from a cathode to an anode. While the technology makes it practical to charge and recharge, the liquid can catch fire if overloaded.
For decades, scientists have seen a potential answer in solid electrolytes, which could allow a battery to soak up more energy without overheating.

If we’re going to get better at powering the planet with renewable energy, we need to get better at finding ways of efficiently storing that energy until it’s needed – and scientists have identified a particular material that could give us exactly that.
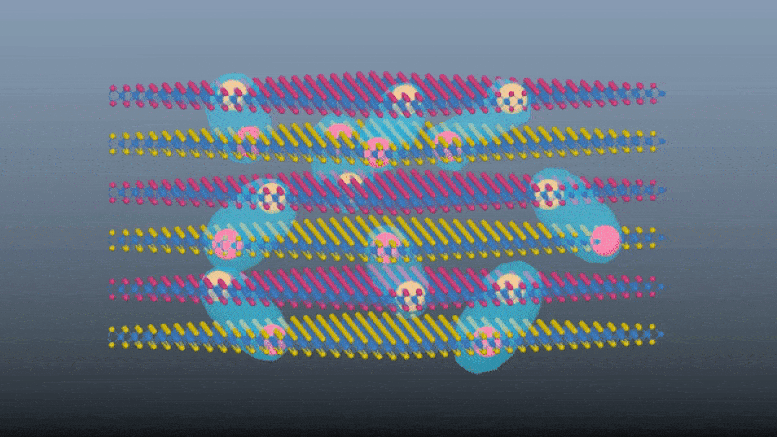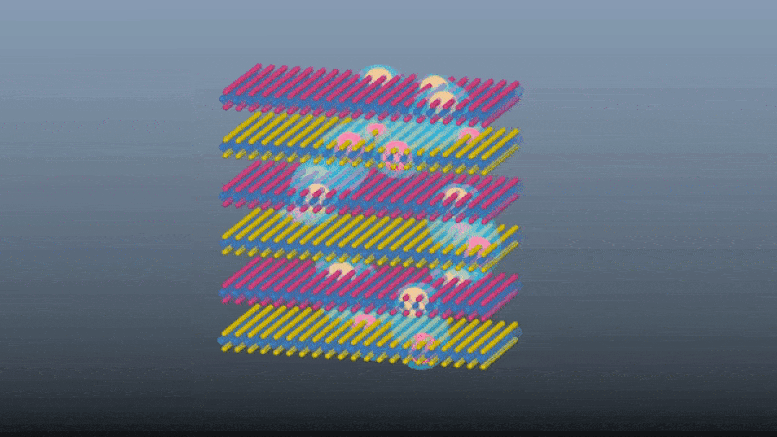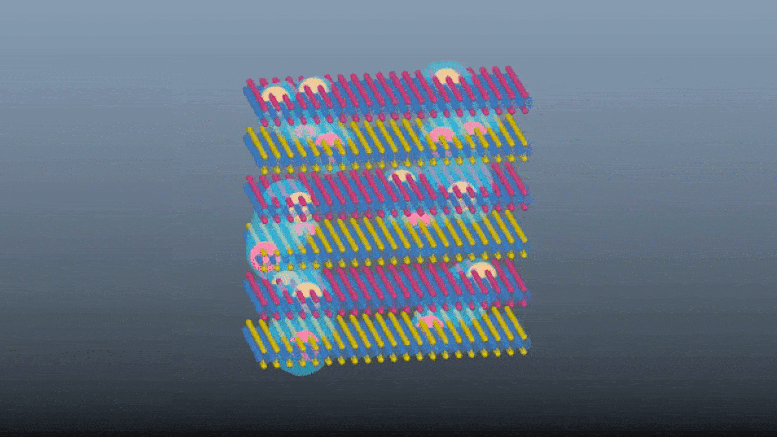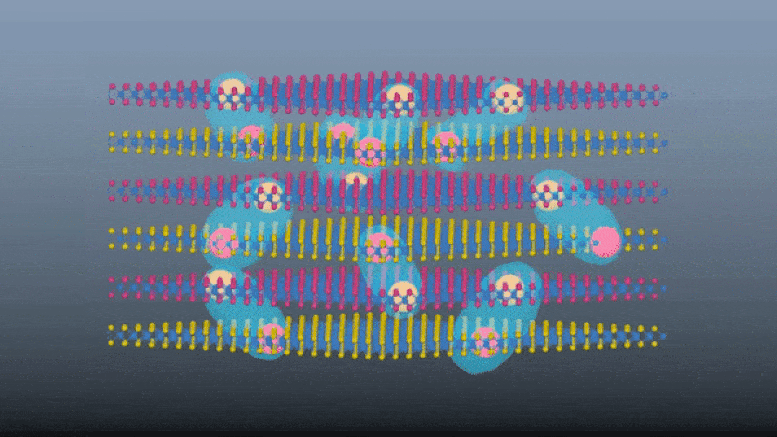
The material is known as a metal-organic framework (MOF), in which carbon-based molecules form structures by linking metal ions. Crucially, MOFs are porous, so they can form composite materials with other small molecules.
That’s what the team did here, adding molecules of the light-absorbing compound azobenzene. The finished composite material was able to store energy from ultraviolet light for at least four months at room temperature before releasing it again – a big improvement over the days or weeks that most light-responsive materials can manage.