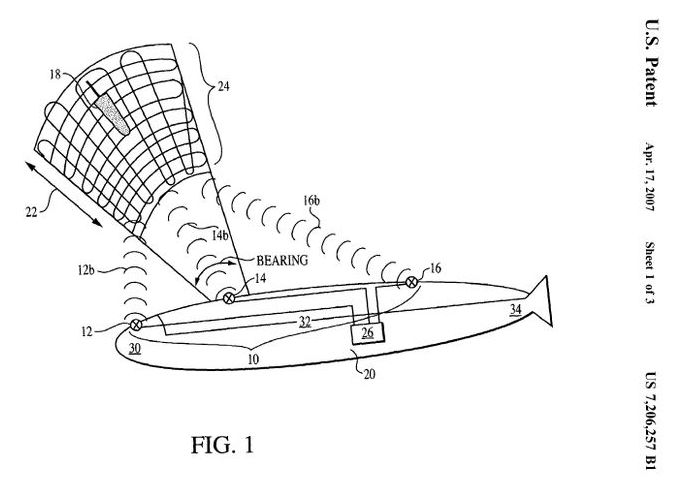
Imagine a day when a submarine could blast a target to smithereens using nothing more than acoustic energy. That’s the idea behind a recently granted U.S. Navy patent for a cavitation weapon. The powerful weapon would use sonar to generate “acoustic remote cavitation,” i.e. a big pressure bubble, that would destroy everything from torpedoes to mines. As the patent describes:
*A method is disclosed of generating a predetermined field of cavitation around a remote target in an underwater environment. The method includes the steps of identifying a remote target location, generating at least two acoustic beams, each at a high power output, from an underwater acoustic source, and controlling the generated acoustic beams to intersect with each other at the remote target location and thereby create a destructive cavitation field at the intersection of the beams. The acoustic source and target can be located in unconfined underwater space and at a distance of at least 100 m apart. *
You’ve read your last complimentary article this month. To read the full article, SUBSCRIBE NOW. If you’re already a subscriber, please sign in and and verify your subscription.