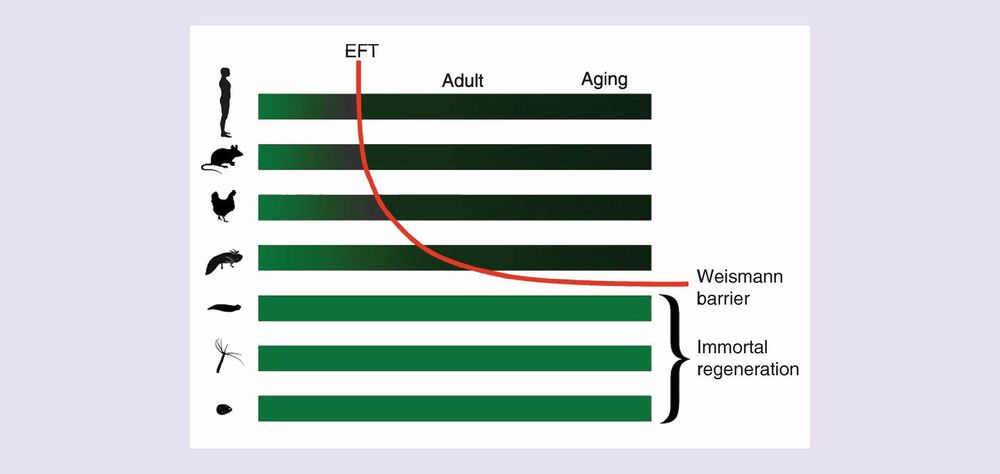
Growing evidence supports the antagonistic pleiotropy theory of mammalian aging. Accordingly, changes in gene expression following the pluripotency transition, and subsequent transitions such as the embryonic–fetal transition, while providing tumor suppressive and antiviral survival benefits also result in a loss of regenerative potential leading to age-related fibrosis and degenerative diseases. However, reprogramming somatic cells to pluripotency demonstrates the possibility of restoring telomerase and embryonic regeneration pathways and thus reversing the age-related decline in regenerative capacity. A unified model of aging and loss of regenerative potential is emerging that may ultimately be translated into new therapeutic approaches for establishing induced tissue regeneration and modulation of the embryo-onco phenotype of cancer.
Keywords:
- acetyl-CoA
- aging
- AMPK
- dietary restriction
- DNA methylation
- epigenetics
- mTOR
- pluripotent stem cells
- regeneration
Aging is often defined as a progressive deterioration of an organism over time, wherein the risk of mortality increases exponentially with age in the postreproductive years. Although everyday environmental risks from predation or infectious disease (e.g., stochastic risks) necessarily lead to increased mortality over time, they are not considered core to the definition of the aging process per se [1,2]. Thus, an important criterion of aging is that it encompasses virtually every somatic tissue type, including the gonads (though not necessarily the germ-line cells themselves, given their role in potentially perpetuating the species) [3]. In order to distinguish the aging process from damage that occurs stochastically over time, Benjamin Gompertz described aging as a process leading to an exponential increase in mortality with time, that is, Rm = R0eat where ‘Rm’ represents the probability of mortality between ages ‘t’ and ‘t + 1’.