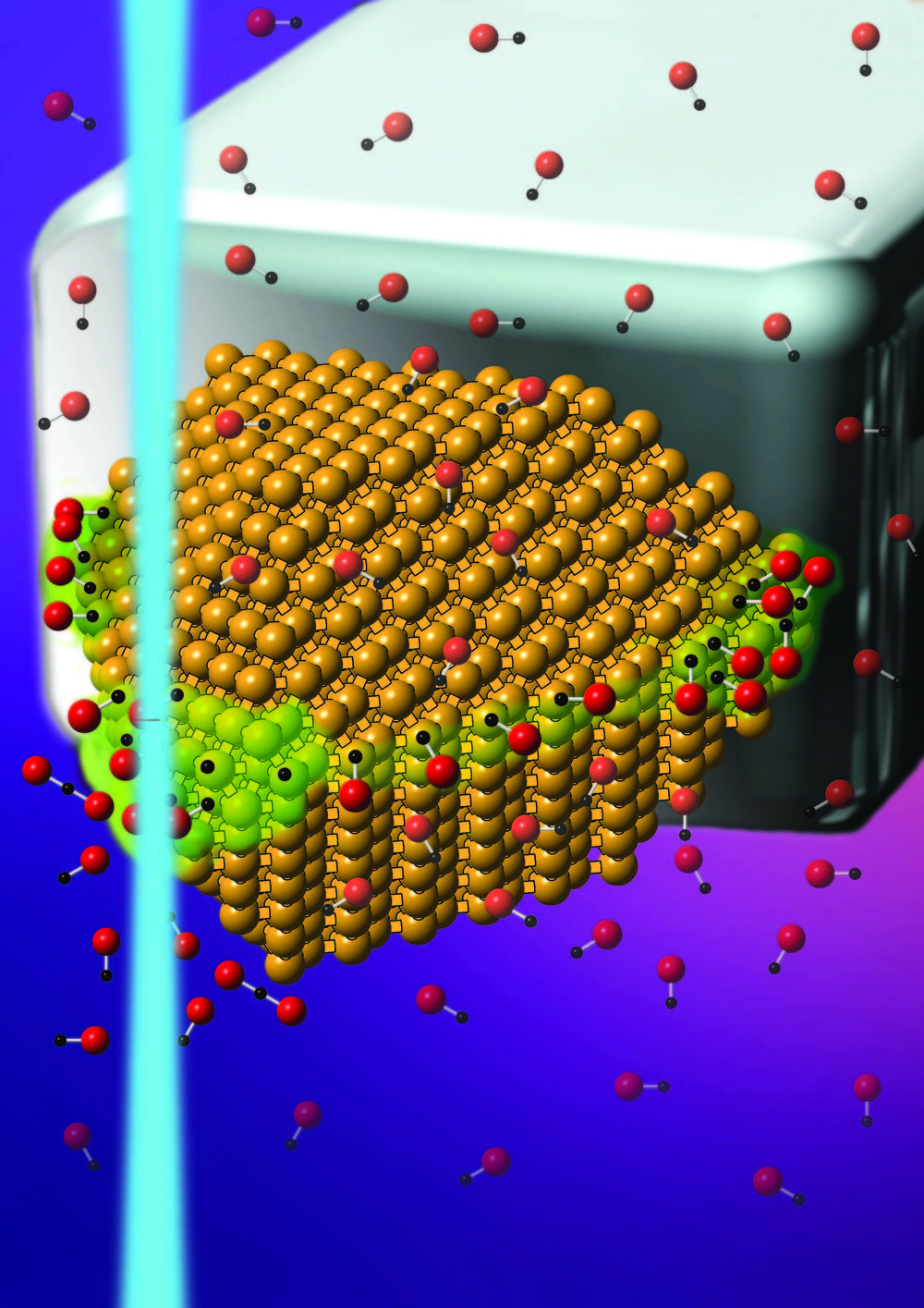
NIST researchers have explored in unprecedented detail a new breed of catalysts that allow some chemical reactions, which normally require high heat, to proceed at room temperature. The energy-saving catalysts use sunlight or another light source to excite localized surface plasmons (LSPs)—oscillations of groups of electrons on the surface of certain metal nanoparticles, such as gold, silver and aluminum. The energy derived from the LSP oscillations drives chemical reactions among molecules that adhere to the nanoparticles.
Scientists had previously shown that molecular hydrogen can be split into its individual atoms by the energy generated by the LSP oscillations. The NIST team has now discovered a second LSP-mediated reaction that proceeds at room temperature. In this reaction, LSPs excited in gold nanoparticles transform two molecules of carbon monoxide into carbon and carbon dioxide. The reaction, which ordinarily requires a minimum temperature of 400 degrees C., plays an important role in converting carbon monoxide into widely used carbon-based materials such as carbon nanotubes and graphite.
Probing the nanoparticles with an electron beam and combining the data with simulations, the NIST researchers pinpointed the sites on the gold nanoparticles where the reactions occurred. They also measured the intensity of the LSPs and mapped how the energy associated with the oscillations varied from place to place inside the nanoparticles. The measurements are key steps in understanding the role of LSPs for initiating reactions at room temperature, mitigating the need to heat the samples.