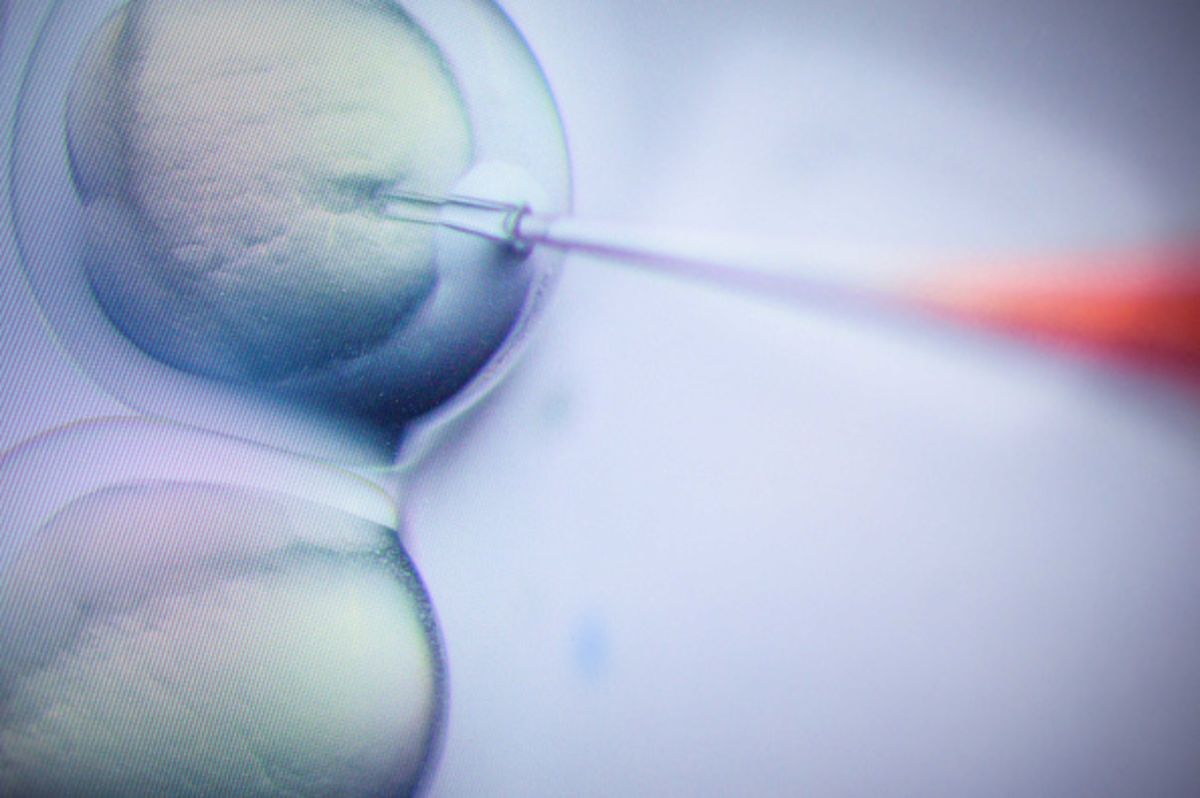
A far better approach, then, is the middle course. Rather than prejudge the products of biotechnology, regulators should screen new plants and single out those that might need special monitoring or restrictions. In the U.S., the Food and Drug Administration does something similar on a voluntary basis for foods made from plants with engineered proteins. Companies submit data about their new products, and if the FDA decides it has no further questions, they can claim their foods are “generally recognized as safe.”
Europe and the U.S. should avoid an all-or-nothing approach to regulating plants made with Crispr.
Read more