Fuel cells and batteries provide electricity by generating and coaxing positively charged ions from a positive to a negative terminal which frees negatively charged electrons to power cellphones, cars, satellites, or whatever else they are connected to. A critical part of these devices is the barrier between these terminals, which must be separated for electricity to flow.
Improvements to that barrier, known as an electrolyte, are needed to make energy storage devices thinner, more efficient, safer, and faster to recharge. Commonly used liquid electrolytes are bulky and prone to shorts, and can present a fire or explosion risk if they’re punctured.
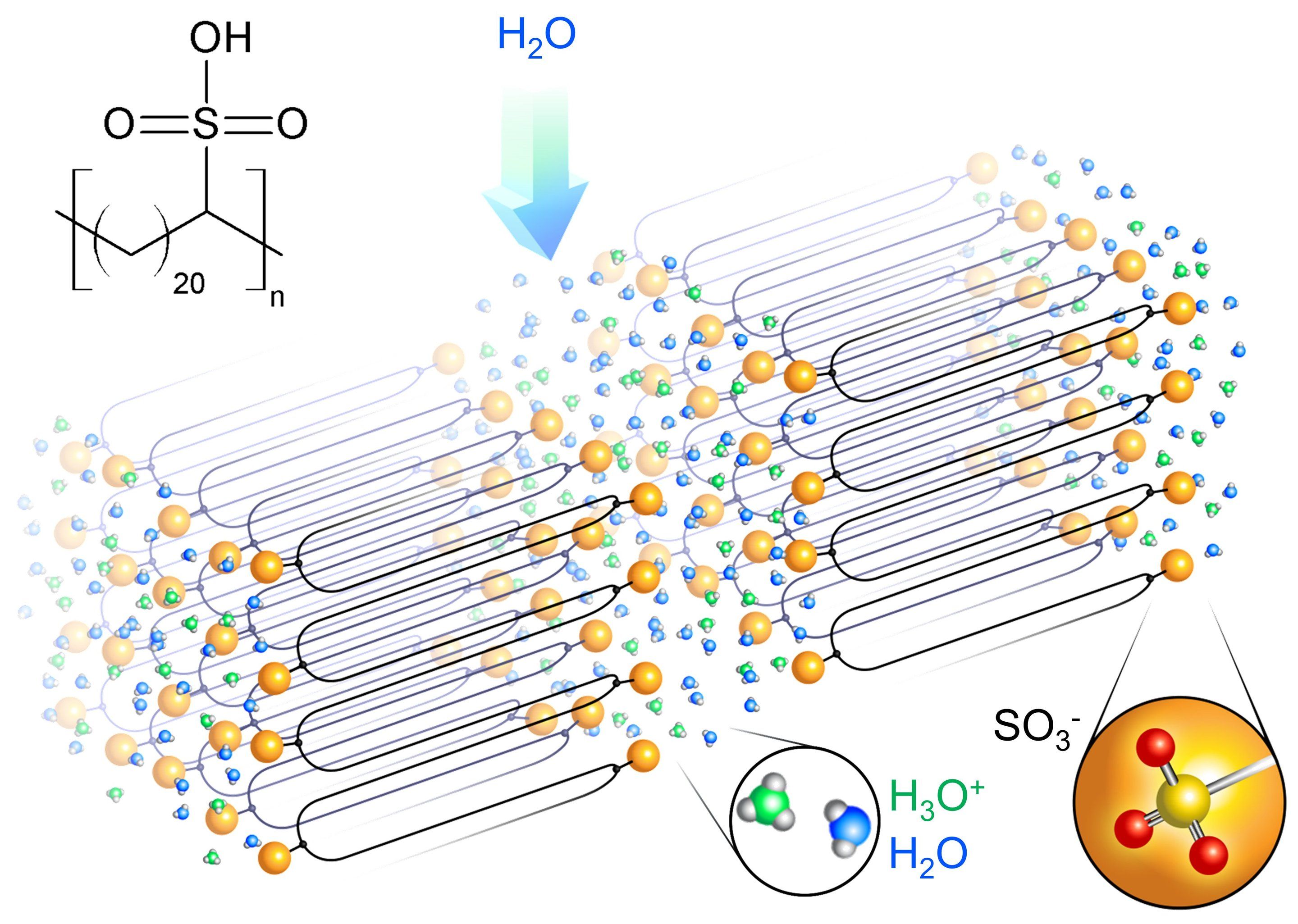
Research led by University of Pennsylvania engineers suggests a different way forward: a new and versatile kind of solid polymer electrolyte (SPE) that has twice the proton conductivity of the current state-of-the-art material. Such SPEs are currently found in proton-exchange membrane fuel cells, but the researchers’ new design could also be adapted to work for the lithium-ion or sodium-ion batteries found in consumer electronics.