Fusion energy has the potential to supply safe, clean, and nearly limitless power. Although fusion reactions can occur for light nuclei weighting less than iron, most elements will not fuse unless they are in the interior of a star. To create burning plasmas in experimental fusion power reactors such as tokamaks and stellarators, scientists seek a fuel that is relatively easy to produce, store, and bring to fusion. The current best bet for fusion reactors is deuterium-tritium fuel. This fuel reaches fusion conditions at lower temperatures compared to other elements and releases more energy than other fusion reactions.
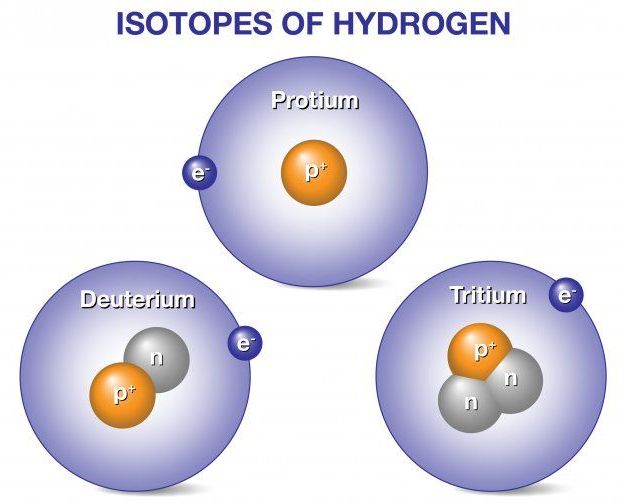
Deuterium and tritium are isotopes of hydrogen, the most abundant element in the universe. Whereas all isotopes of hydrogen have one proton, deuterium also has one neutron and tritium has two neutrons, so their ion masses are heavier than protium, the isotope of hydrogen with no neutrons. When deuterium and tritium fuse, they create a helium nucleus, which has two protons and two neutrons. The reaction releases an energetic neutron. Fusion power plants would convert energy released from fusion reactions into electricity to power our homes, businesses, and other needs.
Fortunately, deuterium is common. About 1 out of every 5000 hydrogen atoms in seawater is in the form of deuterium. This means our oceans contain many tons of deuterium. When fusion power becomes a reality, just one gallon of seawater could produce as much energy as 300 gallons of gasoline.