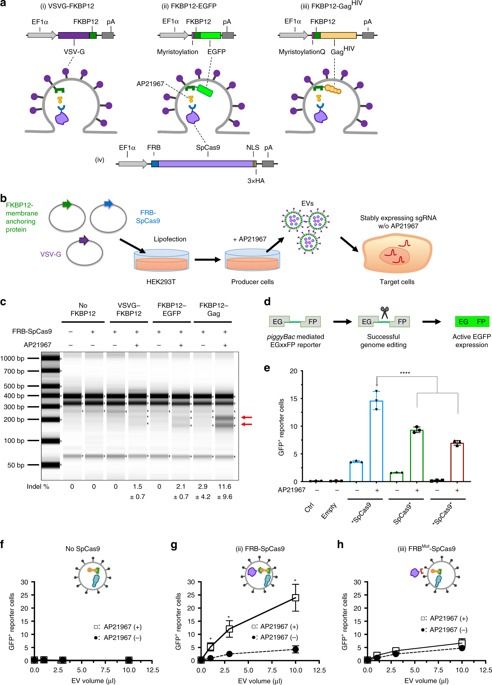
Prolonged expression of the CRISPR-Cas9 nuclease and gRNA from viral vectors may cause off-target mutagenesis and immunogenicity. Thus, a transient delivery system is needed for therapeutic genome editing applications. Here, we develop an extracellular nanovesicle-based ribonucleoprotein delivery system named NanoMEDIC by utilizing two distinct homing mechanisms. Chemical induced dimerization recruits Cas9 protein into extracellular nanovesicles, and then a viral RNA packaging signal and two self-cleaving riboswitches tether and release sgRNA into nanovesicles. We demonstrate efficient genome editing in various hard-to-transfect cell types, including human induced pluripotent stem (iPS) cells, neurons, and myoblasts. NanoMEDIC also achieves over 90% exon skipping efficiencies in skeletal muscle cells derived from Duchenne muscular dystrophy (DMD) patient iPS cells. Finally, single intramuscular injection of NanoMEDIC induces permanent genomic exon skipping in a luciferase reporter mouse and in mdx mice, indicating its utility for in vivo genome editing therapy of DMD and beyond.