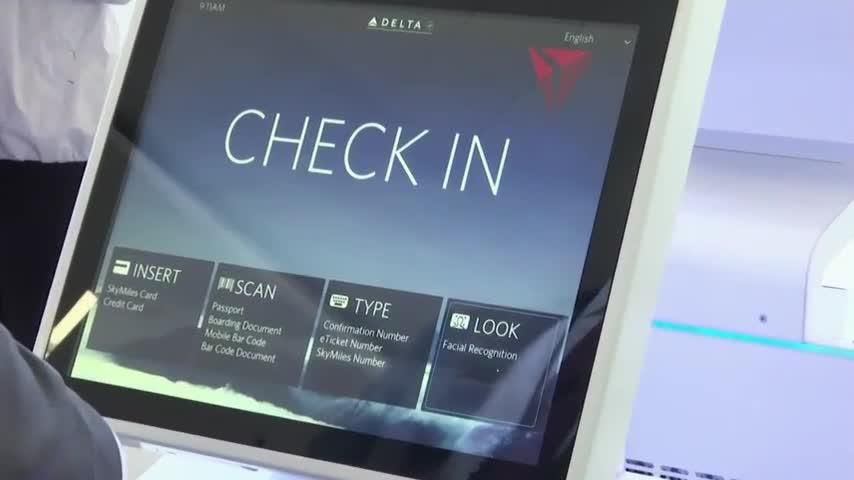
Delta says the Atlanta airport’s Terminal F has become the “first biometric terminal” in the United States where passengers can use facial recognition technology “from curb to gate.”




It’s not like moon-walking astronauts don’t already have plenty of hazards to deal with. There’s less gravity, extreme temperatures, radiation—and the whole place is aggressively dusty. If that weren’t enough, it also turns out that the visual-sensory cues we use to perceive depth and distance don’t work as expected—on the moon, human eyeballs can turn into scam artists.
During the Apollo missions, it was a well-documented phenomenon that astronauts routinely underestimated the size of craters, the slopes of hilltops, and the distance to certain objects. Objects appeared much closer than they were, which created headaches for mission control. Astronauts sometimes overexerted themselves and depleted oxygen supplies in trying to reach objects that were further than expected.
This phenomenon has also become a topic of study for researchers trying to explain why human vision functions differently in space, why so many visual errors occurred, and what, if anything, we can do to prepare the next generation of space travelers.

Ketamine, a drug once associated with raucous parties, bright lights, and loud music, is increasingly being embraced as an alternative depression treatment for the millions of patients who don’t get better after trying traditional medications.
The latest provider of the treatment is Columbia University, one of the nation’s largest academic medical centers.

If classic monster movies and old science experiments are to be believed, life begins with a spark.
Not everybody is convinced by this kind of origin story, so the search continues for sources of energy capable of transforming a prebiotic soup into a life-generating dish. Maybe the secret ingredient isn’t anything more shocking than a pinch of salt.
A new study led by researchers from the Earth-Life Science Institute (ELSI) at Tokyo Institute of Technology in Japan has turned their attention to common old sodium chloride as a potential conduit for the chemical energy required for early biochemistry.

The introduction of the gene editing tool CRISPR in 2007 was a revolution in medical science and cell biology. But even though the potential is great, the launch of CRISPR has been followed by debate about ethical issues and the technology’s degree of accuracy and side effects.
However, in a new study published in Cell, researchers from the Novo Nordisk Foundation Center for Protein Research have described how Cas12a, one of the CRISPR technologies, works at the molecular level. This makes it possible to fine-tune the gene-editing process to achieve specific desired effects.
“If we compare CRISPR to a car engine, what we have done is make a complete 3D map of the engine and thus gained an understanding of how it works. This knowledge will enable us to fine-tune the CRISPR engine and make it work in various ways—as a Formula 1 racer as well as an off-road truck,” says Professor Guillermo Montoya from the Novo Nordisk Foundation Center for Protein Research.

The universe has been making stars for a good 13 billion years or so, and a natural question to ask might be “how many stars have existed in that time?” But now astronomers have taken it several steps further and asked “how much light has been emitted in that time?” Using a new measurement method, the team has apparently managed to quantify all the starlight every produced in the observable universe – and the result is a figure that’ll make your eyes water.
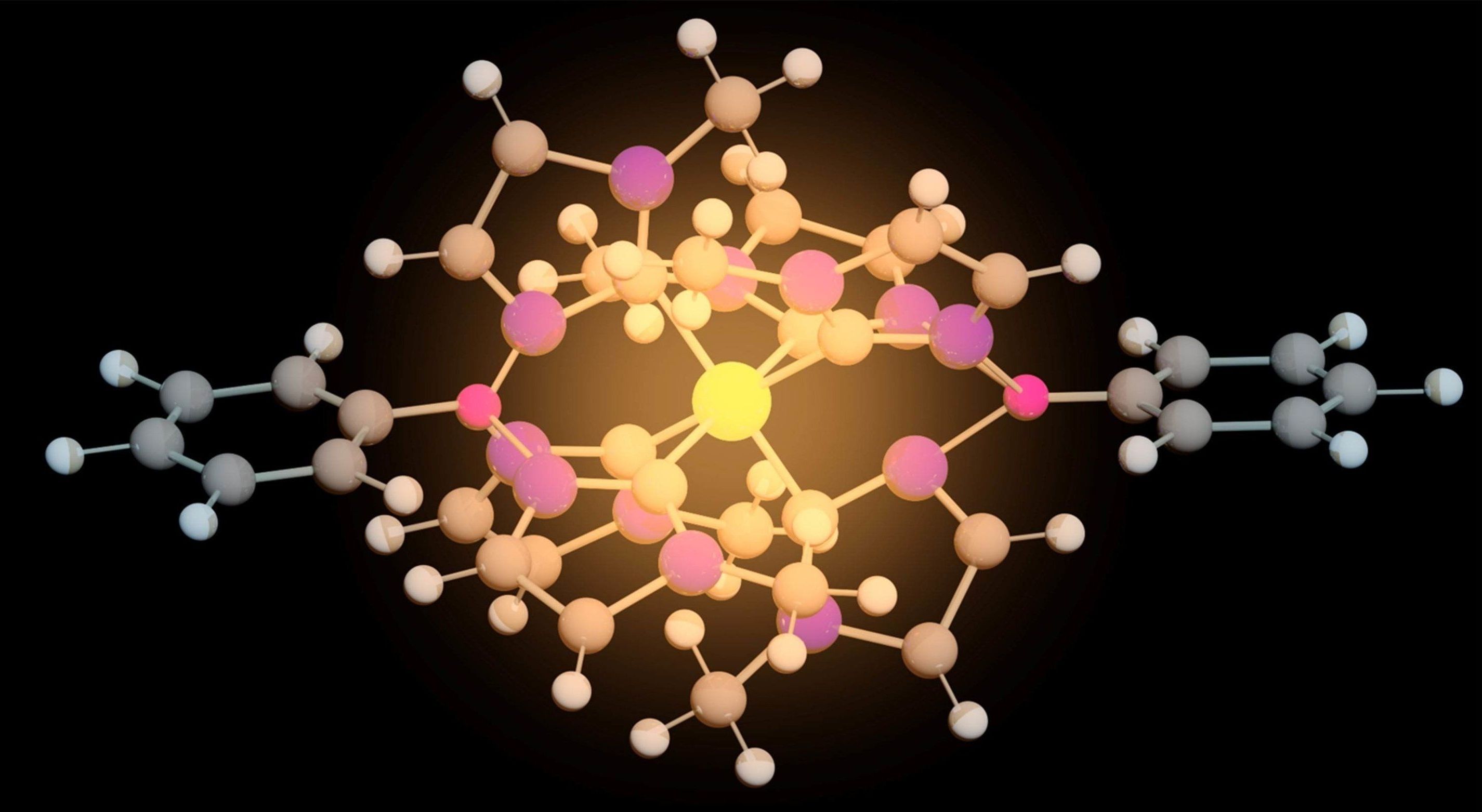
For the first time, researchers have succeeded in creating an iron molecule that can function both as a photocatalyst to produce fuel and in solar cells to produce electricity. The results indicate that the iron molecule could replace the more expensive and rarer metals used today.
Some photocatalysts and solar cells are based on a technology that involves molecules containing metals, known as metal complexes. The task of the metal complexes in this context is to absorb solar rays and utilise their energy. The metals in these molecules pose a major problem, however, as they are rare and expensive metals, such as the noble metals ruthenium, osmium and iridium.
“Our results now show that by using advanced molecule design, it is possible to replace the rare metals with iron, which is common in the Earth’s crust and therefore cheap,” says Chemistry Professor Kenneth Wärnmark of Lund University in Sweden.